|
Sumanene
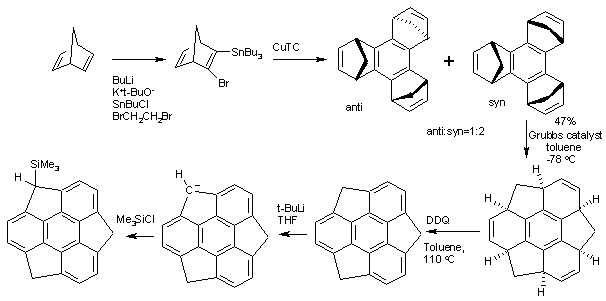
Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the molecule can be considered a fragment of buckminsterfullerene. ''Suman'' means "sunflower" in both Hindi and Sanskrit. The core of the arene is a benzene ring and the periphery consists of alternating benzene rings (3) and cyclopentadiene rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reactions. Organic synthesis The structure of Sumanene can be inferred from oxidation of 1,5,9-trimethyltriphenylene but the first practical synthesis starts from norbornadiene. Norbornadiene is converted into a stannane by action of ''n''-butyllithium, dibromoethane and tributyltinchloride. An Ullmann reaction of this stannane with CuTC affords the benzene core. The methylene bridges (--) created in this conversion then migrate in a tandem ring-opening metathesis and ring-closing metathesis by the Grubbs' catalyst. The final structure is obtained by oxidation by DDQ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sumanene Synthesis
Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the molecule can be considered a fragment of buckminsterfullerene. ''Suman'' means "sunflower" in both Hindi and Sanskrit. The core of the arene is a benzene ring and the periphery consists of alternating benzene rings (3) and cyclopentadiene rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reactions. Organic synthesis The structure of Sumanene can be inferred from oxidation of 1,5,9-trimethyltriphenylene but the first practical synthesis starts from norbornadiene. Norbornadiene is converted into a stannane by action of ''n''-butyllithium, dibromoethane and tributyltinchloride. An Ullmann reaction of this stannane with CuTC affords the benzene core. The methylene bridges (--) created in this conversion then migrate in a tandem ring-opening metathesis and ring-closing metathesis by the Grubbs' catalyst. The final structure is obtained by oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbornadiene
Norbornadiene is an organic compound In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ... and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerization, isomerize (isomers would be anti-Bredt alkenes). Norbornadiene is also a useful Diels–Alder_reaction#The_dienophile, dienophile in Diels-Alder reactions. Synthesis Norbornadiene can be formed by a Diels-Alder reaction between cyclopentadiene and acetylene : Reactions Quadricyclane, a valence isomer, can be obtained from norbornadiene by a photochemical reaction when assisted by a photochemical sensitizer, sensiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid. Preparation Synthesis of DDQ involves cyanation of chloranil. J. Thiele and F. Günther first reported a 6-step preparation in 1906. The substance did not receive interest until its potential as a dehydrogenation agent was discovered. A single-step chlorination from 2,3-dicyanohydroquinone was reported in 1965. Reactions The reagent removes pairs of H atoms from organic molecules. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene: :2 C6Cl2(CN)2O2 + C10H12 → 2 C6Cl2(CN)2(OH)2 + C10H8 The resulting hydroquinone is poorly soluble in typical reaction solvents (dioxane, benzene, alkanes), which facilitates workup. Solutions of DDQ in benzene are red, due to the formation of a charge-tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grubbs' Catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis. First-generation catalyst In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use. The first well-defined ruthenium catalyst was reported in 1992. It was prepared from RuCl2(PPh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring-closing Metathesis
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the ''E-'' or ''Z-'' isomers and volatile ethylene.Carey, F. A.; Sunburg, R. J. Reactions Involving Transition Metals. ''Advanced Organic Chemistry: Reaction and Synthesis'', 5th Ed.; Part B; Springer: New York, 2010, pp. 761-767.Monfette, S.; Fogg, D. E. (2009). "Equilibrium Ring-Closing Metathesis". ''Chem. Rev.'' 109 (8): 3783-3816. . The most commonly synthesized ring sizes are between 5-7 atoms;Deiters, A.; Martin, S. F. (2004). “Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis”. ''Chem. Rev.'' 104 (5): 2199-2238. . however, reported syntheses include 45- up to 90- membered macroheterocycles.Cain, M. F.; Forrest, W. P.; Peryshkov, R. V.; Schrock, R. R. Muller, P. (2013). “Synthesis of a TREN in Whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring-opening Metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry. Catalysts The reaction requires metal catalysts. Most commercially important processes employ heterogeneous catalysts. The heterogeneous catalysts are often prepared by in-situ activation of a metal halides (MClx) using organoaluminium or organotin compounds, e.g. combining MClx–EtAlCl2. A typical catalyst support is alumina. Commercial catalysts are often based on molybdenum and ruthenium. Well-defined organometallic compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tandem Reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the previous step.Tietze, L. F.; Beifuss, U. ''Angew. Chem. Int. Ed.'' 1993, ''32'', 131–163. In cascade reactions, isolation of intermediates is not required, as each reaction composing the sequence occurs spontaneously. In the strictest definition of the term, the reaction conditions do not change among the consecutive steps of a cascade and no new reagents are added after the initial step.Padwa, A.; Bur, S. K. ''Tetrahedron'' 2007, ''63'', 5341–5378. By contrast, one-pot procedures similarly allow at least two reactions to be carried out consecutively without any isolation of intermediates, but do not preclude the addition of new reagents or the change of conditions after the first reaction. Thus, any cascade reaction is also a one-pot p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Bridges
Methylene may refer to: * Methylene group or methylene bridge (CH2< or equivalently -CH2-), a part of a molecule connected to the rest of the molecule by two single bonds. * An older name for methylidene (=CH2), a part of a molecule connected to another atom by a double bond. * Methylene (compound) (CH2), an organic compound. See also * Bichloride of methylene (30% methanol and 70% chloroform), a variant of the old anesthetic A.C.E. mixture * Methyl group * Methylenedioxy * Mytilene, a city in Greece * Methanol Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ... {{disambiguation de:Methylengruppe sv:Metylengrupp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I)-thiophene-2-carboxylate
Copper(I) thiophene-2-carboxylate or CuTC is a coordination complex derived from copper and thiophene-2-carboxylic acid. It is used as a reagent to promote the Ullmann reaction between aryl In organic chemistry, an aryl is any functional group or substituent derived from an aromaticity, aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar ... halides. : References {{reflist Thiophenes Copper(I) compounds Reagents for organic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann Reaction
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides. Traditionally this reaction is effected by copper, but palladium and nickel are also effective catalysts. The reaction is named after Fritz Ullmann. Mechanism The mechanism of the Ullmann reaction is extensively studied. Complications arise because the reactions are often heterogeneous. With copper as the halide acceptor, organocopper intermediates are invoked. Scope A typical example of classic Ullmann biaryl coupling is the conversion of ''ortho''-chloronitrobenzene into 2,2'-dinitrobiphenyl with a copper - bronze alloy. : : The traditional version of the Ullmann reaction requires harsh reaction conditions, and the reaction has a reputation for erratic yields. Because of these problems many improvements and alternative procedures have been introduced. The classical Ullmann reaction is limited to electron deficient aryl halides and requires harsh reaction conditions. Modern variants of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibromoethane
Dibromoethane can refer to either of two isomeric organobromides with the molecular formula C2H4Br2: * 1,1-Dibromoethane (ethylidene dibromide) * 1,2-Dibromoethane 1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a ... (ethylene dibromide) See also * Dibromoethene {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |