Sumanene Synthesis on:
[Wikipedia]
[Google]
[Amazon]
Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
can be considered a fragment of buckminsterfullerene. ''Suman'' means "sunflower
The common sunflower (''Helianthus annuus'') is a large annual forb of the genus ''Helianthus'' grown as a crop for its edible oily seeds. Apart from cooking oil production, it is also used as livestock forage (as a meal or a silage plant), as ...
" in both Hindi
Hindi (Devanāgarī: or , ), or more precisely Modern Standard Hindi (Devanagari: ), is an Indo-Aryan language spoken chiefly in the Hindi Belt region encompassing parts of northern, central, eastern, and western India. Hindi has been de ...
and Sanskrit
Sanskrit (; attributively , ; nominally , , ) is a classical language belonging to the Indo-Aryan branch of the Indo-European languages. It arose in South Asia after its predecessor languages had diffused there from the northwest in the late ...
. The core of the arene is a benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
ring and the periphery
Periphery or peripheral may refer to:
Music
*Periphery (band), American progressive metal band
* ''Periphery'' (album), released in 2010 by Periphery
* "Periphery", a song from Fiona Apple's album '' The Idler Wheel...''
Gaming and entertainm ...
consists of alternating benzene rings (3) and cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often ab ...
rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
s.
Organic synthesis
The structure of Sumanene can be inferred fromoxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of 1,5,9-trimethyltriphenylene
Triphenylene is an organic compound with the formula (C6H4)3. A flat polycyclic aromatic hydrocarbon (PAH), it consists of four fused benzene rings. Triphenylene has delocalized 18-''π''-electron systems based on a planar structure, correspondin ...
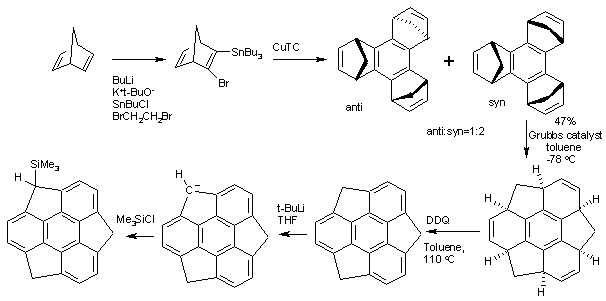
but the first practical synthesis starts from norbornadiene. Norbornadiene is converted into a stannane by action of ''n''-butyllithium, dibromoethane Dibromoethane can refer to either of two isomeric organobromides with the molecular formula C2H4Br2:
* 1,1-Dibromoethane (ethylidene dibromide)
* 1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine comp ...
and tributyltinchloride. An Ullmann reaction of this stannane with CuTC affords the benzene core. The methylene bridges (--) created in this conversion then migrate in a tandem ring-opening metathesis and ring-closing metathesis
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the ''E-' ...
by the Grubbs' catalyst. The final structure is obtained by oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
by DDQ.
:
Properties
Sumanene is a bowl-shapedmolecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
with a bowl depth of 118 picometers. The 6 hub carbon atoms are pyramidalized by 9° and the molecule displays considerable bond alternation (138.1 to 143.1 pm). Sumanene also experiences bowl-to-bowl inversion with an inversion barrier In chemistry, pyramidal inversion (also umbrella inversion) is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion passin ...
of 19.6 kcal/ mol (82 kJ/mol) at 140 °C which is much higher than that found for its corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20 H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is irculene. It is of scientific interest because it is a geodesic ...
cousin.
Like any benzylic proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
, the sumanene protons can be abstracted by a strong base such as t-butyl lithium to form the sumanene mono carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
. This strong nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
can react with an electrophile such as trimethylsilyl chloride to the trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is ch ...
derivative.
The trianion has also been reported. Electron transport properties have been investigated as well as carbon NMR Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is ...
Derivatives
Sumanene derivatives such as ''naphtosumanene'' and ''trisialsumanene'' have been described. Chiral sumanenes are of some interest with respect toinherent chirality
In chemistry, inherent chirality is a property of asymmetry in molecules arising, not from a stereogenic or chiral center, but from a twisting of the molecule in 3-D space. The term was first coined by Volker Boehmer in a 1994 review, to describ ...
, examples are chiral ''trimethylsumanene'' and a chiral sumanene cyclopentadienyl iron complex Sakane, H., Amaya, T., Moriuchi, T. and Hirao, T. (2009), A Chiral Concave-Bound Cyclopentadienyl Iron Complex of Sumanene. Angewandte Chemie International Edition, 48: 1640–1643.
References
{{Reflist Geodesic polyarenes Polycyclic aromatic hydrocarbons