|
Selenite (ion)
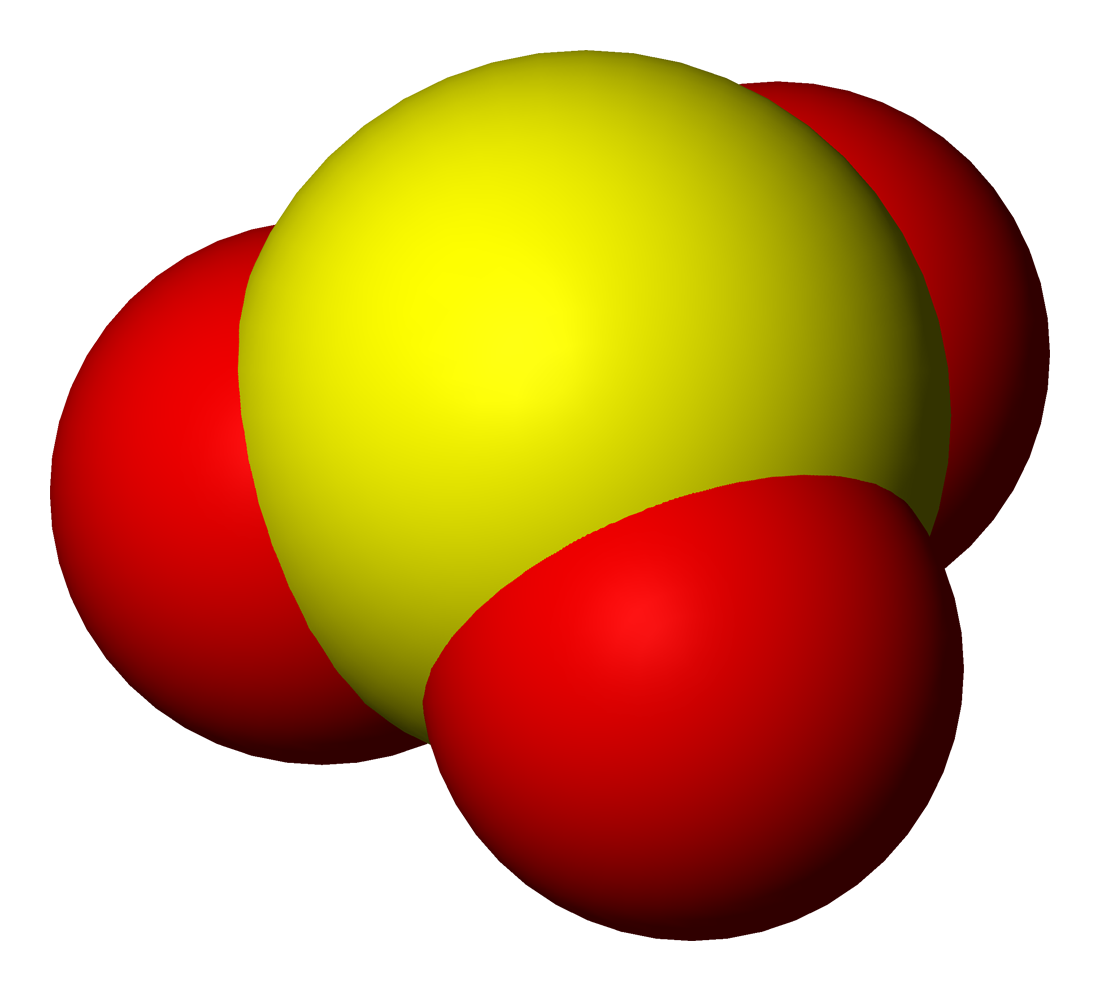
Selenite refers to the anion with the chemical formula . It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, . Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite which is a common source of selenite. Selenite also refers to the esters of selenous acid, for example dimethyl selenite . Synthesis and reactions Selenite salts can be prepared by neutralizing solutions of selenous acid, which is generated by dissolving selenium dioxide in water. The process proceeds via the hydrogenselenite ion, . Selenite reacts with elemental sulfur to form thioselenate: : Most selenite salts can be formed by heating the metal oxide with selenium dioxide Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium. Properties Solid SeO2 is a one-dimensional polymer, the chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenites
Selenite may refer to: Substances containing selenium *A selenium-containing anion or ionic compound with the SeO32− anion: **Selenite (ion), anion is a selenium oxoanion with the chemical formula SeO32− ***Selenous acid, the conjugate acid, with the formula H2SeO3 ***Salts of this anion: **** Silver selenite, an inorganic compound of formula Ag2SeO3 ****Sodium selenite, a salt, a colourless solid, and the most common water-soluble selenium compound *Selenite broth, an enrichment medium for the isolation of ''Salmonella'' species Other * Selenite (mineral), a variety of gypsum (CaSO4·2H2O) * ''Selenites'' is a synonym of ''Zophos'', a genus of land snails in the family Haplotrematidae * Selenite Range, a mountain range in Pershing County, Nevada ** Selenite Peak, a summit in the Selenite Range, Nevada * Selenite, a high class citizen that experienced space travel, from the videogame ''Event_0'' * Selenite, a fictional native inhabitant of the Moon; see Moon in science ficti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyanion
An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound . The structures of condensed oxyanions can be rationalized in terms of AO''n'' polyhedral units with sharing of corners or edges between polyhedra. The oxyanions (specifically, phosphate and polyphosphate esters) adenosine monophosphate ( AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology. Monomeric oxyanions The formula of monomeric oxyanions, , is dictated by the oxidation state of the element A and its position in the periodic table. Elements of the first row are limited to a maximum coordination number of 4. However, none of the first row elements has a monomeric oxyanion with that coordination number. Instead, ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Selenite
Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound. Synthesis and fundamental reactions Sodium selenite usually is prepared by the reaction of selenium dioxide with sodium hydroxide: :SeO2 + 2 NaOH → Na2SeO3 + H2O The hydrate converts to the anhydrous salt upon heating to 40 °C. According to X-ray crystallography, both anhydrous Na2SeO3 and its pentahydrate feature pyramidal SeO32−. The Se-O distances range from 1.67 to 1.72 Å. Oxidation of this anion gives sodium selenate, Na2SeO4. Applications Together with the related barium and zinc selenites, sodium selenite is mainly used in the manufacture of colorless glass. The pink color imparted by these selenites cancels out the green color imparted by iron impurities. Because selenium is an essential element, sodium selenite is an ingredient in dietary supplements such as multi-v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |