|
Saturated
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds ** Saturated and unsaturated compounds **Degree of unsaturation ** Saturated fat or fatty acid ** Unsaturated fat or fatty acid * Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mutagenesis * Saturation (genetic), the observed number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Fat
A saturated fat is a type of fat in which the fatty acid chains have all single bonds. A fat known as a glyceride is made of two kinds of smaller molecules: a short glycerol backbone and fatty acids that each contain a long linear or branched chain of carbon (C) atoms. Along the chain, some carbon atoms are linked by single bonds (-C-C-) and others are linked by double bonds (-C=C-). A double bond along the carbon chain can react with a pair of hydrogen atoms to change into a single -C-C- bond, with each H atom now bonded to one of the two C atoms. Glyceride fats without any carbon chain double bonds are called saturated because they are "saturated with" hydrogen atoms, having no double bonds available to react with more hydrogen. Most animal fats are saturated. The fats of plants and fish are generally unsaturated. Various foods contain different proportions of saturated and unsaturated fat. Many processed foods like foods deep-fried in hydrogenated oil and sausage are high in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturation (commutative Algebra)
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds **Saturated and unsaturated compounds **Degree of unsaturation **Saturated fat or fatty acid **Unsaturated fat or fatty acid * Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Enzyme kinetics#General principles, Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygenation (medicine), Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
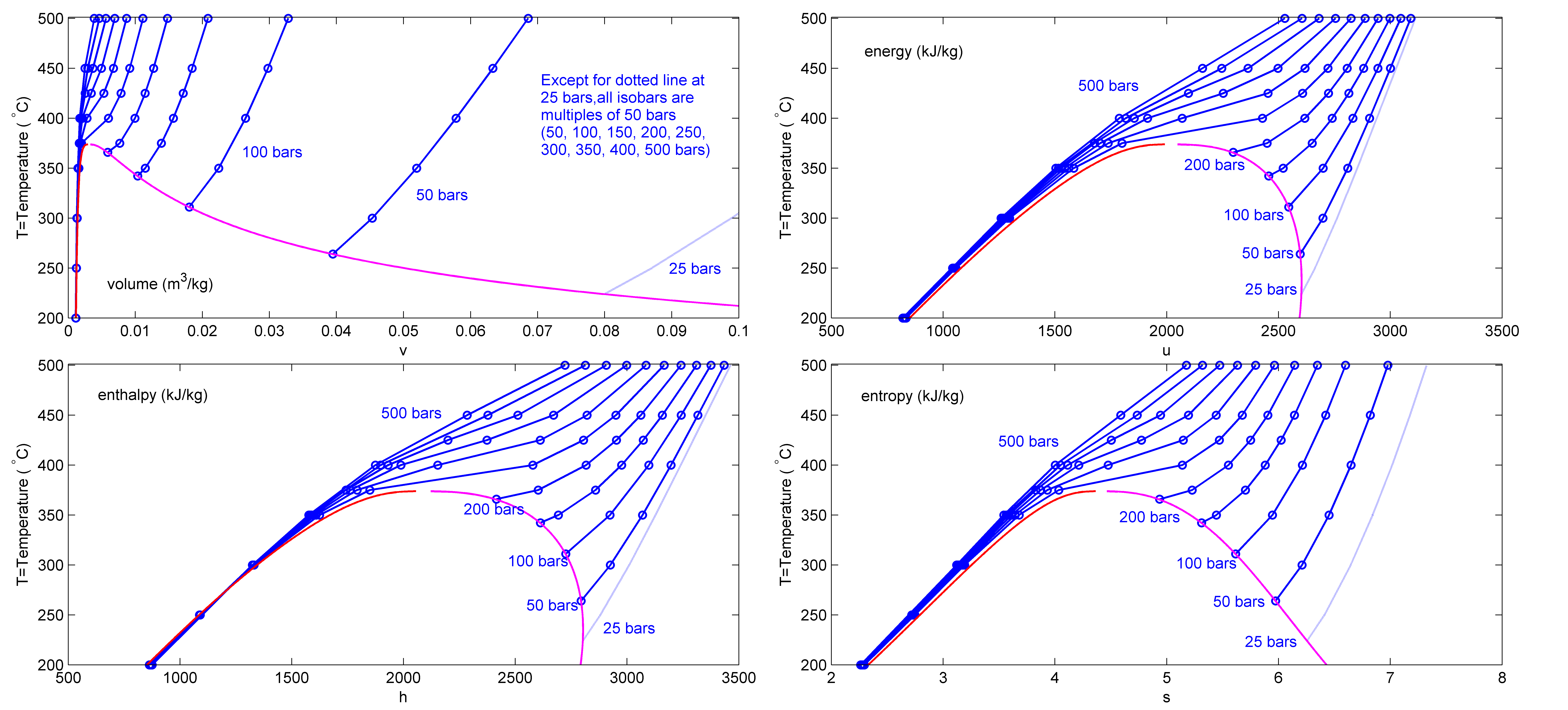
Saturated Fluid
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds ** Saturated and unsaturated compounds **Degree of unsaturation ** Saturated fat or fatty acid **Unsaturated fat or fatty acid * Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mutagenesis * Saturation (genetic), the observed number of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superheated Steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured. Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its temperature without changing state (i.e., condensing) from a gas, to a mixture of saturated vapor and liquid. If unsaturated steam (a mixture which contains both water vapor and liquid water droplets) is heated at constant pressure, its temperature will also remain constant as the vapor quality (think dryness, or percent saturated vapor) increases towards 100%, and becomes dry (i.e., no saturated liquid) saturated steam. Continued heat input will then "super" heat the dry saturated steam. This will occur if saturated steam contacts a surface with a higher temperature. Superheated steam and liquid water cannot coexist under thermodynamic equilibrium, as any additional heat simply evaporates more water and the steam will become saturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Content
Water content or moisture content is the quantity of water contained in a material, such as soil (called soil moisture), rock, ceramics, crops, or wood. Water content is used in a wide range of scientific and technical areas, and is expressed as a ratio, which can range from 0 (completely dry) to the value of the materials' porosity at saturation. It can be given on a volumetric or mass (gravimetric) basis. Definitions Volumetric water content, θ, is defined mathematically as: :\theta = \frac where V_w is the volume of water and V_\text = V_s + V_w + V_a is equal to the total volume of the wet material, i.e. of the sum of the volume of solid host material (e.g., soil particles, vegetation tissue) V_s, of water V_w, and of air V_a. Gravimetric water content is expressed by mass (weight) as follows: :u = \frac where m_w is the mass of water and m_s is the mass of the solids. For materials that change in volume with water content, such as coal, the gravimetric water content, ''u' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis acids and bases, Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'. Organic chemistry Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols. Unsaturated organic compounds The concept of saturation can be described using various naming systems, formulas, and Analytical chemistry, analytical tests. For instance, IUPAC nomenclature of organic chemistry, IU ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Absorption
In experimental atomic physics, saturated absorption spectroscopy or Doppler-free spectroscopy is a set-up that enables the precise determination of the transition frequency of an atom between its ground state and an optically excited state. The accuracy to which these frequencies can be determined is, ideally, limited only by the width of the excited state, which is the inverse of the lifetime of this state. However, the samples of atomic gas that are used for that purpose are generally at room temperature, where the measured frequency distribution is highly broadened due to the Doppler effect. Saturated absorption spectroscopy allows precise spectroscopy of the atomic levels without having to cool the sample down to temperatures at which the Doppler broadening is no longer relevant (which would be on the order of a few millikelvins). It is also used to lock the frequency of a laser to the precise wavelength of an atomic transition in atomic physics experiments. Doppler broadening ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Mutagenesis
Site saturation mutagenesis (SSM), or simply site saturation, is a random mutagenesis technique used in protein engineering, in which a single codon or set of codons is substituted with all possible amino acids at the position. There are many variants of the site saturation technique, from paired site saturation (saturating two positions in every mutant in the library) to scanning site saturation (performing a site saturation at every site in the protein, resulting in a library of size 0 x (number of residues in the protein)that contains every possible point mutant of the protein). Method Saturation mutagenesis is commonly achieved by site-directed mutagenesis PCR with a randomised codon in the primers (e.g. SeSaM) or by artificial gene synthesis, with a mixture of synthesis nucleotides used at the codons to be randomised. Different degenerate codons can be used to encode sets of amino acids. Because some amino acids are encoded by more codons than others, the exact rati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unsaturated Fat
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond. Where double bonds are formed, hydrogen atoms are subtracted from the carbon chain. Thus, a saturated fat has no double bonds, has the maximum number of hydrogens bonded to the carbons, and therefore is "saturated" with hydrogen atoms. In cellular metabolism, unsaturated fat molecules contain somewhat less energy (i.e., fewer calories) than an equivalent amount of saturated fat. The greater the degree of unsaturation in a fatty acid (i.e., the more double bonds in the fatty acid) the more vulnerable it is to lipid peroxidation (rancidity). Antioxidants can protect unsaturated fat from lipid peroxidation. Chemistry and nutrition Double bonds may be in either a ''cis'' or a ''trans'' isomer, depending on the geometry of the double b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degree Of Unsaturation
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of rings and π bonds. A formula is used in organic chemistry to help draw chemical structures. It does not give any information about those components individually—the specific number of rings, or of double bonds (one π bond each), or of triple bonds (two π bonds each). The final structure is verified with use of NMR, mass spectrometry and IR spectroscopy, as well as qualitative inspection. It is based on comparing the actual molecular formula to what would be a possible formula if the structure were saturated—having no rings and containing only σ bonds—with all atoms having their standard valence. General formula The formula for degree of unsaturation is: :\mathrm = 1 + \tfrac \sum n_i(v_i-2) where ''ni'' is the number of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dew Point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will condense to form liquid water known as dew. When this occurs via contact with a colder surface, dew will form on that surface. The dew point is affected by humidity. When there is more moisture in the air, the dew point is higher. When the temperature is below the freezing point of water, the dew point is called the frost point, as frost is formed via deposition rather than condensation. In liquids, the analog to the dew point is the cloud point. Humidity If all the other factors influencing humidity remain constant, at ground level the relative humidity rises as the temperature falls; this is because less vapor is needed to saturate the air. In normal conditions, the dew point temperature will not be greater than the air temperature, sinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Kinetics
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier ( inhibitor or activator) might affect the rate. An enzyme (E) is typically a protein molecule that promotes a reaction of another molecule, its substrate (S). This binds to the active site of the enzyme to produce an enzyme-substrate complex ES, and is transformed into an enzyme-product complex EP and from there to product P, via a transition state ES*. The series of steps is known as the mechanism: : E + S ⇄ ES ⇄ ES* ⇄ EP ⇄ E + P This example assumes the simplest case of a reaction with one substrate and one product. Such cases exist: for example, a mutase such as phosphoglucomutase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |