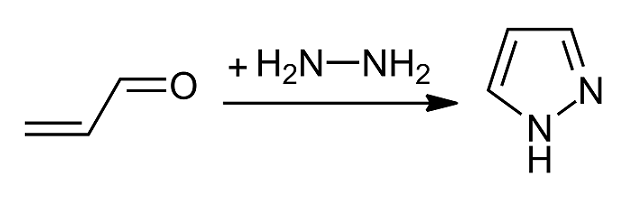|
Pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by Germa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles Synthesis
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by Germa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trispyrazolylborate
In inorganic chemistry, the trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion B(C3N2H3)3sup>−, but the term trispyrazolylborate refers to derivatives substituted at on the pyrazolyl rings. This family of compounds are sometimes called scorpionate ligands. Tp ligands As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetry. The facial bonding mode is reminiscent of cyclopentadienyl ligands, although the ligand field stabilization energy of Tp− is weaker as indicated by the fact that Fe(Tp)2 is a spin-crossover complex whereas ferrocene is low-spin. The Tp ligands are usually prepared from the reaction of pyrazole with potassium borohydride: :KBH4 + 3 C3H3N2H → K B(C3N2H3)3 + 3H2 Intermediates include the monopyrazolylbor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scorpionate Ligand
In coordination chemistry, the term scorpionate ligand refers to a tridentate (three-donor-site) ligand which would bind to a metal in a ''fac'' manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then little-known DuPont chemist of Ukrainian descent, Swiatoslaw Trofimenko. Trofimenko called this discovery "a new and fertile field of remarkable scope". The term scorpionate comes from the fact that the ligand can bind a metal with two donor sites like the pincers of a scorpion; the third and final donor site reaches over the plane formed by the metal and the other two donor atoms to bind to the metal. The binding can be thought of as being like a scorpion grabbing the metal with two pincers before stinging it. While many scorpionate ligands are of the Tp class, many other scorpionate ligands are known. For example, the Tm and tripod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celecoxib
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour. Common side effects include abdominal pain, nausea, and diarrhea. Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis. Use is not recommended in people at high risk for heart disease. The risks are similar to other NSAIDs, such as ibuprofen and naproxen. Use in the later part of pregnancy or during breastfeeding is not recommended. Celecoxib was patented in 1993 and came into medical use in 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine Hydrate
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ludwig Knorr
Ludwig Knorr (2 December 1859 – 4 June 1921) was a German chemist. Together with Carl Paal, he discovered the Paal–Knorr synthesis, and the Knorr quinoline synthesis and Knorr pyrrole synthesis are also named after him. The synthesis in 1883 of the analgesic drug antipyrine, now called phenazone, was a commercial success. Antipyrine was the first synthetic drug and the most widely used drug until it was replaced by Aspirin in the early 20th century. Early life Ludwig Knorr was born to a wealthy merchant family in 1859. He grew up in the Sabbadini-Knorr company headquarters, located in the Kaufingerstraße in the center of Munich, and in the family house near the Lake Starnberg. After the early death of his father, the education of him and his four brothers lay in the hands of their mother. In 1878 Knorr received his Abitur and started to study chemistry at the University of Munich. In the beginning, he studied under Jacob Volhard; then, after Volhard left for the Universit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, di ketones, di esters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans Von Pechmann
Hans von Pechmann (1 April 1850 – 19 April 1902) was a German chemist, renowned for his discovery of diazomethane in 1894. Pechmann condensation and Pechmann pyrazole synthesis. He also first prepared 1,2-diketones (e.g., diacetyl), acetonedicarboxylic acid, methylglyoxal and diphenyltriketone; established the symmetrical structure of anthraquinone. Von Pechmann also produced the first example of solid polyethylene serendipitously in 1898, via the decomposition of diazomethane. He was born in Nürnberg. After studying with Heinrich Limpricht at the University of Greifswald he became professor at the University of Munich till 1895. He was professor at the University of Tübingen from 1895 until his death. He killed himself by taking cyanide, aged 52. Works * ''Volhard's Anleitung zur Qualitativen chemischen Analyse'' . Chemisches Labolatorium des Staates, München 9th & 10th ed. 190Digital editionby the University and State Library Düsseldorf * ''Anleitung zur quantitativen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stanozolol
Stanozolol ( abbrev. Stz), sold under many brand names, is an androgen and anabolic steroid (AAS) medication derived from dihydrotestosterone (DHT). It is used to treat hereditary angioedema. It was developed by American pharmaceutical company Winthrop Laboratories (Sterling Drug) in 1962, and has been approved by the U.S. Food and Drug Administration for human use, though it is no longer marketed in the USA. It is also used in veterinary medicine. Stanozolol has mostly been discontinued, and remains available in only a few countries. It is given by mouth in humans or by injection into muscle in animals. Unlike most injectable AAS, stanozolol is not esterified and is sold as an aqueous suspension, or in oral tablet form. The drug has a high oral bioavailability, due to a C17α alkylation which allows the hormone to survive first-pass liver metabolism when ingested. It is because of this that stanozolol is also sold in tablet form. Stanozolol is one of the AAS commonly used as p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds. Properties Tautomerism The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tridentate Ligand
A tridentate ligand (or terdentate ligand) is a ligand that has three atoms that can function as acceptor atoms in a coordination complex. Well-known tridentate ligands include diethylenetriamine with three nitrogen donor atoms, and the iminodiacetate anion which consists of one deprotonated amine nitrogen and a pair of carboxylate groups. An octahedrally coordinated atom has six positions around it. Two tridentate ligands may form a complex with such an atom. There are two possible arrangements for such a complex: ''fac'' where the coordination is in a triangle on one face of the octahedron, and ''mer'' where the coordinating atoms are in an arc around the central atom, with two atoms of the ligand opposite each other. ''Fac'' tridentate ligands are termed scorpionate ligands, especially in reference to polypyrazolylborates. If the tridentate ligand is not symmetrical, then in the ''fac'' complexes in octahedral coordination there are three possible isomers. In the ''mer'' co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |