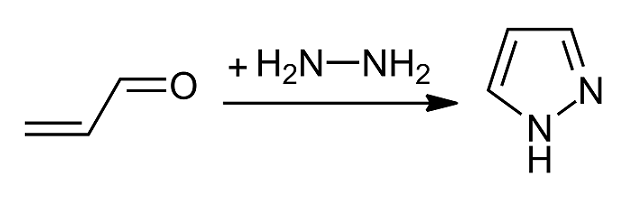|
Pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ... atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles Synthesis
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by German ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scorpionate Ligand
In coordination chemistry, the term scorpionate ligand refers to a tridentate (three-donor-site) ligand which would bind to a metal in a ''fac'' manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then little-known DuPont chemist of Ukrainian descent, Swiatoslaw Trofimenko. Trofimenko called this discovery "a new and fertile field of remarkable scope". The term scorpionate comes from the fact that the ligand can bind a metal with two donor sites like the pincers of a scorpion; the third and final donor site reaches over the plane formed by the metal and the other two donor atoms to bind to the metal. The binding can be thought of as being like a scorpion grabbing the metal with two pincers before stinging it. While many scorpionate ligands are of the Tp class, many other scorpionate ligands are known. For example, the Tm and tripod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celecoxib
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour. Common side effects include abdominal pain, nausea, and diarrhea. Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis. Use is not recommended in people at high risk for heart disease. The risks are similar to other NSAIDs, such as ibuprofen and naproxen. Use in the later part of pregnancy or during breastfeeding is not recommended. Celecoxib was patented in 1993 and came into medical use in 1999 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stanozolol
Stanozolol (Abbreviation, abbrev. Stz), sold under many brand names, is an androgen and anabolic steroid (AAS) medication derived from dihydrotestosterone (DHT). It is used to treat hereditary angioedema. It was developed by American pharmaceutical company Sterling Drug, Winthrop Laboratories (Sterling Drug) in 1962, and has been approved by the U.S. Food and Drug Administration for human use, though it is no longer marketed in the USA. It is also used in veterinary medicine. Stanozolol has mostly been discontinued, and remains available in only a few countries. It is given Oral administration, by mouth in humans or by Intramuscular injection, injection into muscle in animals. Unlike most injectable AAS, stanozolol is not esterified and is sold as an Aqueous solution, aqueous suspension, or in oral tablet form. The drug has a high oral bioavailability, due to a C17α alkylation which allows the hormone to survive first-pass liver metabolism when ingested. It is because of this th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate and triethylborohydride or triethylhydroborate . Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table). Tetrahydroborates are also of academic and industrial interest in inorganic chemistry. History Alkali metal borohydrides were first described in 1940 by Hermann Irving Schlesinger and Herbert C. Brown. They synthesized lithium borohydride from diborane : :, where M = Li, Na, K, Rb, Cs, etc. Current methods involve reduction of trimethyl borate with sodium hydride. Structure In the borohydride anion and most of its modifications, boron has a tetrahedral structure. The reactivity of the B− ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tridentate Ligand
A tridentate ligand (or terdentate ligand) is a ligand that has three atoms that can function as acceptor atoms in a coordination complex. Well-known tridentate ligands include diethylenetriamine with three nitrogen donor atoms, and the iminodiacetate anion which consists of one deprotonated amine nitrogen and a pair of carboxylate groups. An octahedrally coordinated atom has six positions around it. Two tridentate ligands may form a complex with such an atom. There are two possible arrangements for such a complex: ''fac'' where the coordination is in a triangle on one face of the octahedron, and ''mer'' where the coordinating atoms are in an arc around the central atom, with two atoms of the ligand opposite each other. ''Fac'' tridentate ligands are termed scorpionate ligands, especially in reference to polypyrazolylborates. If the tridentate ligand is not symmetrical, then in the ''fac'' complexes in octahedral coordination there are three possible isomers. In the ''mer'' com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trispyrazolylborate
In inorganic chemistry, the trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion B(C3N2H3)3sup>−, but the term trispyrazolylborate refers to derivatives substituted at on the pyrazolyl rings. This family of compounds are sometimes called scorpionate ligands. Tp ligands As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetry. The facial bonding mode is reminiscent of cyclopentadienyl ligands, although the ligand field stabilization energy of Tp− is weaker as indicated by the fact that Fe(Tp)2 is a spin-crossover complex whereas ferrocene is low-spin. The Tp ligands are usually prepared from the reaction of pyrazole with potassium borohydride: :KBH4 + 3 C3H3N2H → K B(C3N2H3)3 + 3H2 Intermediates include the monopyrazolylborate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine Hydrate
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Scorpionate
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcone
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. Chemical properties Chalcones have two absorption maxima at 280 nm and 340 nm. Synthesis Chalcone is usually prepared by an aldol condensation between benzaldehyde and acetophenone. : This reaction, which can be carried out without any solvent, is so reliable that it is used in as an example of green chemistry in undergraduate education. Biosynthesis Chalcones and chalconoids are synthesized in plants as secondary metabolites. The enzyme chalcone synthase, a type III polyketide synthase, is responsible for the biosynthesis of these compounds. The enzyme is found in all "higher" (vascular) and several "lower" ( non-vascular) plants. Potential pharmacology Chalcones and their derivatives demonstrate a wide range of biological activities including anti-inflammation. Some 2′-amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |