|
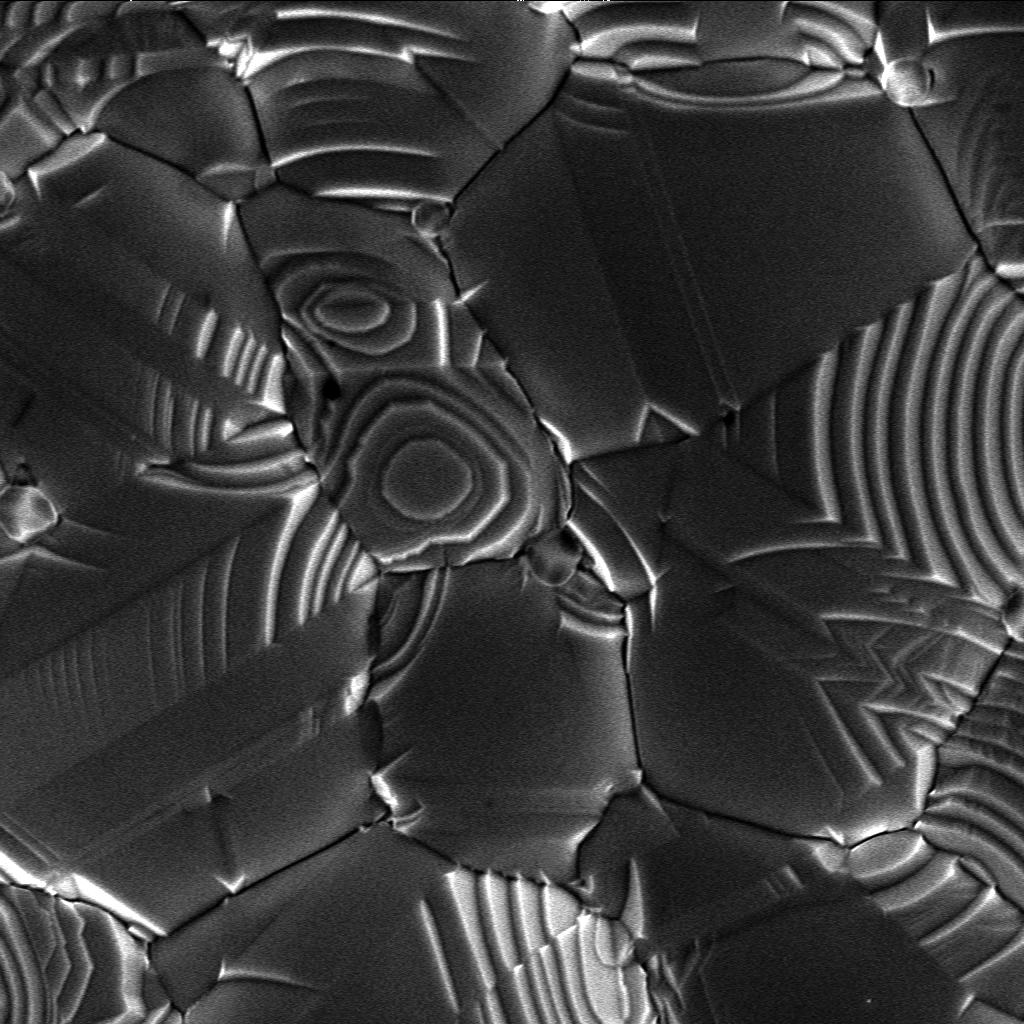
Protocrystalline
A protocrystalline phase is a distinct phase occurring during crystal growth which evolves into a microcrystalline form. The term is typically associated with silicon films in optical applications such as solar cells. Applications Silicon solar cells Amorphous silicon (a-Si) is a popular solar cell material owing to its low cost and ease of production. Owing to disordered structure ( Urbach tail), its absorption extends to the energies below the band gap resulting in a wide-range spectral response; however, it has a relatively low solar cell efficiency. Protocrystalline Si (pc-Si:H) also has a relatively low absorption near the band gap owing to its more ordered crystalline structure. Thus, protocrystalline and amorphous silicon can be combined in a tandem solar cell where the top thin layer of a-Si:H absorbs short-wavelength light whereas the longer wavelengths are absorbed by the underlying protocrystalline silicon layer. See also *Amorphous silicon *Crystallite * Multiju ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs. Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films onto a variety of flexible substrates, such as glass, metal and plastic. Amorphous silicon cells generally feature low efficiency. As a second-generation thin-film solar cell technology, amorphous silicon was once expected to become a major contributor in the fast-growing worldwide photovoltaic market, but has since lost its significance due to strong competition from conventional crystalline silicon cells and other thin-film technologies such as CdTe and CIGS. Amorphous silicon is a preferred material for the thin film transistor (TFT) elements of liquid crystal displays (LCDs) and for x-ray imagers. Amorphous silicon differs from other allotropic variations, such as monocrystalline silicon—a single crystal, and polycrystalline silicon, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space relative to each other. The crystalline state of matter is characterized by a distinct structural rigidity and very high resistance to deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus and of the shear modulus of elasticity. This contrasts w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcrystalline
A microcrystalline material is a crystallized substance or rock that contains small crystals visible only through microscopic examination. There is little agreement on the range of crystal sizes that should be regarded as microcrystalline, but the extreme range of values suggested is 1 to 200 microns. See also * Macrocrystalline * Nanocrystalline silicon * Microcrystalline cellulose * Microcrystalline wax * Protocrystalline * Rock microstructure Rock microstructure includes the texture and small-scale structures of a rock. The words ''texture'' and ''microstructure'' are interchangeable, with the latter preferred in modern geological literature. However, ''texture'' is still acceptable b ... References Mineralogy concepts Petrology concepts {{Petrology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Cell
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.Solar Cells chemistryexplained.com It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as , , or resistance, vary when exposed to light. Individual solar cell devices are often the electrical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optics
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultraviolet, and infrared light. Because light is an electromagnetic wave, other forms of electromagnetic radiation such as X-rays, microwaves, and radio waves exhibit similar properties. Most optical phenomena can be accounted for by using the classical electromagnetic description of light. Complete electromagnetic descriptions of light are, however, often difficult to apply in practice. Practical optics is usually done using simplified models. The most common of these, geometric optics, treats light as a collection of rays that travel in straight lines and bend when they pass through or reflect from surfaces. Physical optics is a more comprehensive model of light, which includes wave effects such as diffraction and interference that cannot be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urbach Energy
The Urbach Energy, or Urbach Edge, is a parameter typically denoted E_, with dimensions of energy, used to quantify energetic disorder in the band edges of a semiconductor. It is evaluated by fitting the absorption coefficient as a function of energy to an exponential function. It is often used to describe electron transport in structurally disordered semiconductors such a hydrogenated amorphous silicon. Introduction In the simplest description of a semiconductor, a single parameter is used to quantify the onset of optical absorption: the band gap, E_. In this description, semiconductors are described as being able to absorb photons above E_, but are transparent to photons below E_. However, the density of states in 3 dimensional semiconductors increases further from the band gap (this is not generally true in lower dimensional semiconductors however). For this reason, the absorption coefficient, \alpha, increases with energy. The Urbach Energy quantifies the ''steepness'' of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Cell Efficiency
Solar-cell efficiency refers to the portion of energy in the form of sunlight that can be converted via photovoltaics into electricity by the solar cell. The efficiency of the solar cells used in a photovoltaic system, in combination with latitude and climate, determines the annual energy output of the system. For example, a solar panel with 20% efficiency and an area of 1 m2 will produce 200 kWh/yr at Standard Test Conditions if exposed to the Standard Test Condition solar irradiance value of 1000 W/m2 for 2.74 hours a day. Usually solar panels are exposed to sunlight for longer than this in a given day, but the solar irradiance is less than 1000 W/m2 for most of the day. A solar panel can produce more when the sun is high in the sky and will produce less in cloudy conditions or when the sun is low in the sky, usually the sun is lower in the sky in the winter. Two location dependant factors that affect solar PV efficiency are the dispersion and intensity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random Texture (chemistry), texture, or directed, possibly due to growth and processing conditions. While the structure of a (single crystal, single) crystal is highly ordered and its crystal lattice, lattice is continuous and unbroken, Amorphous solid, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in-between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multijunction
Multi-junction (MJ) solar cells are solar cells with multiple p–n junctions made of different semiconductor materials. Each material's p-n junction will produce electric current in response to different wavelengths of light. The use of multiple semiconducting materials allows the absorbance of a broader range of wavelengths, improving the cell's sunlight to electrical energy conversion efficiency. Traditional single-junction cells have a maximum theoretical efficiency of 33.16%. Theoretically, an infinite number of junctions would have a limiting efficiency of 86.8% under highly concentrated sunlight. As of 2008 the best lab examples of traditional crystalline silicon (c-Si) solar cells had efficiencies between 20% and 25%, while lab examples of multi-junction cells have demonstrated performance over 46% under concentrated sunlight. Commercial examples of tandem cells are widely available at 30% under one-sun illumination, and improve to around 40% under concentrated sunlight. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA). Structure Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Polycarbonates received their name because they are polymers containing carbonate groups (−O−(C=O)−O−). A balance of useful features, including temperature resistance, impact resistance and o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |