|
Pleuromutilin Antibiotic
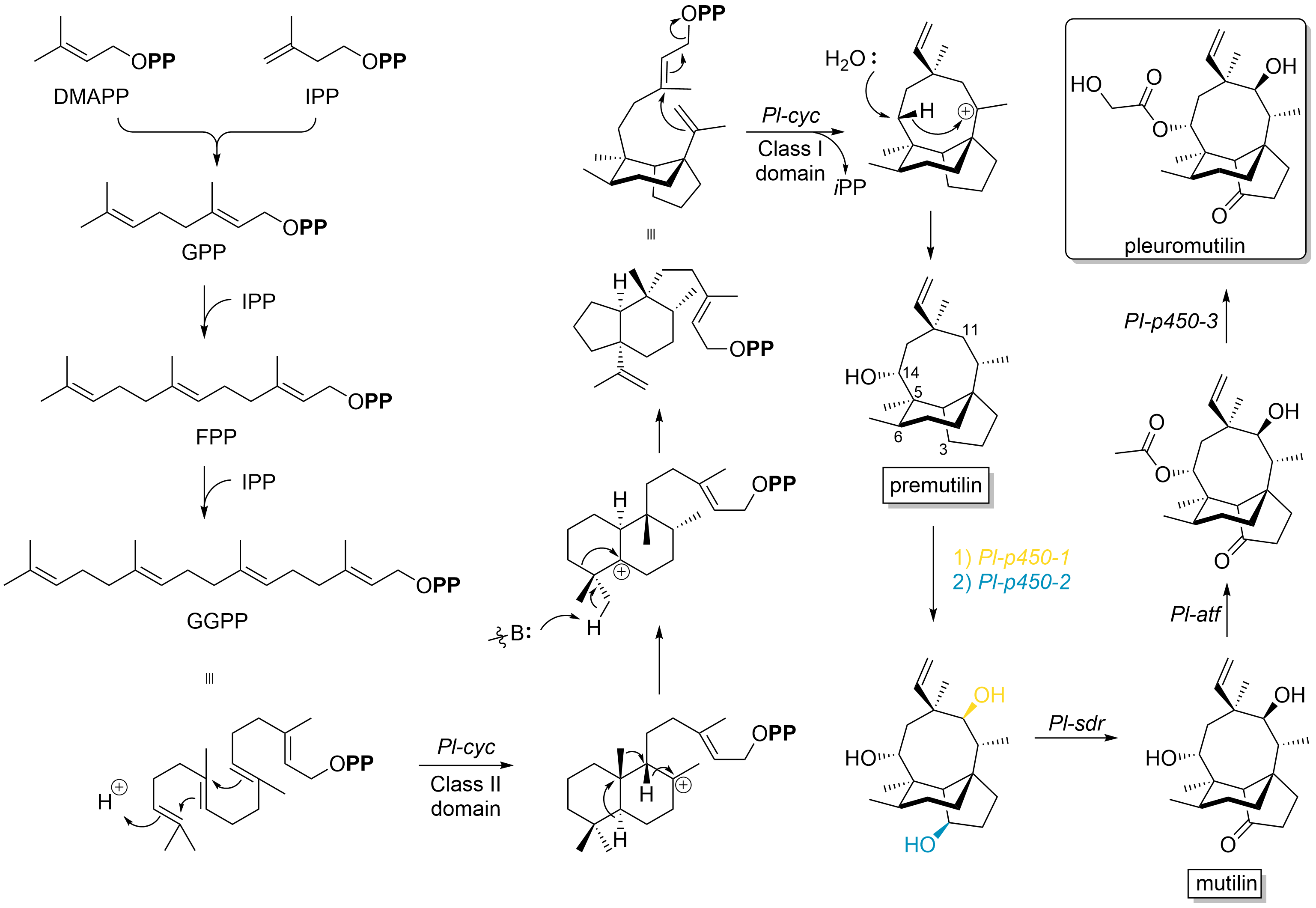
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi '' Omphalina mutila'' (formerly ''Pleurotus mutilus'') and ''Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in '' Drosophila subatrata'', '' Clitopilus scyphoides'', and some other ''Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate pathway (ME ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Synthesis
Protein biosynthesis (or protein synthesis) is a core biological process, occurring inside Cell (biology), cells, homeostasis, balancing the loss of cellular proteins (via Proteolysis, degradation or Protein targeting, export) through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences. Protein synthesis can be divided broadly into two phases - Transcription (biology), transcription and Translation (biology), translation. During transcription, a section of DNA encoding a protein, known as a gene, is converted into a template molecule called messenger RNA (mRNA). This conversion is carried out by enzymes, known as RNA polymerases, in the cell nucleus, nucleus of the cell. In eukaryotes, this mRNA is initially produced in a premature form (Primary transcript, pre-mRNA) which undergoes post-tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clitopilus Scyphoides
''Clitopilus'' is a genus of fungi in the family Entolomataceae. The genus has a widespread distribution, especially in northern temperate areas. Although a 2008 estimate suggested about 30 species in the genus, a more recent publication (2009) using molecular phylogenetics has redefined the genus to include many former ''Rhodocybe'' species. Species *''Clitopilus acerbus'' Noordel. & Co-David *''Clitopilus albovelutinus'' (G. Stev.) Noordel. & Co-David *''Clitopilus alutaceus'' (Singer) Noordel. & Co-David *'' Clitopilus amarellus'' (Cons., D. Antonini, M. Antonini & Contu) Noordel. & Co-David *''Clitopilus amygdaliformis'' Zhu L. Yang *''Clitopilus angustisporus'' (Singer) Noordel. & Co-David *''Clitopilus ardosiacus'' (E. Horak & Griesser) Noordel. & Co-David *''Clitopilus aureicystidiatus'' (Lennox ex T.J. Baroni) Noordel. & Co-David *''Clitopilus australis'' (Singer) Noordel. & Co-David *''Clitopilus azalearum'' (Murrill) Noordel. & Co-David *''Clitopilus balearicus'' (Courte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutilin
Retapamulin is a topical antibiotic developed by GlaxoSmithKline. It is the first drug in the new class of pleuromutilin antibiotics to be approved for human use. It is marketed as an ointment under the brand names Altabax and Altargo. Retapamulin was approved by the United States Food and Drug Administration in April 2007 for the treatment of bacterial skin infections such as impetigo. In May 2007, retapamulin received approval in the EU from the European Medicines Agency for the same indication. Clinical trials have demonstrated its efficacy against certain Gram-positive bacteria including MRSA. Indications Retapamulin is indicated for the topical treatment of impetigo due to '' Staphylococcus aureus'' (methicillin-susceptible only) or ''Streptococcus pyogenes''. Pharmacology Mechanism of action Retapamulin is an antibacterial agent, specifically a protein synthesis inhibitor A protein synthesis inhibitor is a compound that stops or slows the growth or proliferation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Short-chain Dehydrogenase
The short-chain dehydrogenases/reductases family (SDR) is a very large family of enzymes, most of which are known to be NAD- or NADP-dependent oxidoreductases. As the first member of this family to be characterised was Drosophila alcohol dehydrogenase, this family used to be called 'insect-type', or 'short-chain' alcohol dehydrogenases. Most members of this family are proteins of about 250 to 300 amino acid residues. Most dehydrogenases possess at least 2 domains, the first binding the coenzyme, often NAD, and the second binding the substrate. This latter domain determines the substrate specificity and contains amino acids involved in catalysis. Little sequence similarity has been found in the coenzyme binding domain although there is a large degree of structural similarity, and it has therefore been suggested that the structure of dehydrogenases has arisen through gene fusion of a common ancestral coenzyme nucleotide sequence with various substrate specific domains. Subfamilies * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance (pharmacology), clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Ronald W. Estabrook, Estabrook, David Y. Cooper, Cooper, and Otto Rosenthal, Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of secondary metabolite, defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdom (biology), kingdoms of life: animals, plants, fungus, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleuromutilin Proposed Cyclisation Mechanism
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi '' Omphalina mutila'' (formerly ''Pleurotus mutilus'') and ''Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in '' Drosophila subatrata'', '' Clitopilus scyphoides'', and some other ''Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate pathway ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. Howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decalin
Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. Isomers Decalin occurs in ''cis'' and ''trans'' forms. The ''trans'' form is energetically more stable because of fewer steric interactions. ''cis''-Decalin is a chiral molecule without a chiral center; it has a two-fold rotational symmetry axis, but no reflective symmetry. However, the chirality is canceled through a chair-flipping process that turns the molecule into its mirror image. Image:Cis-trans isomerism of decahydronaphthalene.svg, Image:cis-decalin double chair.png, 2: Image:trans-decalin double chair.png, 3: File:Cisdecalin conformations.png, 4: ''trans''-Decalin The only possible way to join the two six-membered rings in the ''trans'' position means the second ring needs to start from two equatorial bonds (blue) of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeranyl Pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, which is its primary use in human cells. It is formed from farnesyl pyrophosphate by the addition of an isoprene unit from isopentenyl pyrophosphate. In ''Drosophila'', geranylgeranyl pyrophosphate is synthesised by HMG-CoA encoded by the Columbus gene. Geranylgeranyl pyrophosphate is utilised as a chemoattractant for migrating germ cells that have traversed the midgut epithelia. The attractant signal is produced at the gonadal precursors, directing the germ cells to these sites, where they will differentiate into eggs and spermatozoa (sperm). Related compounds * Farnesyl pyrophosphate * Geranylgeraniol * Geranyl pyrophosphate Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mevalonate Pathway
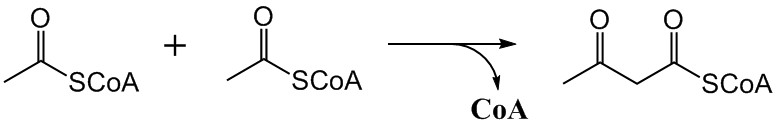
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful. Often, the aim is to discover a new route of synthesis for a target molecule for which there already exist known routes. Sometimes, however, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |