Carbocation on:
[Wikipedia]
[Google]
[Amazon]


 A carbocation is an ion with a positively charged
A carbocation is an ion with a positively charged
 He dubbed the relationship between color and salt formation halochromy, of which malachite green is a prime example.
Carbocations are reactive intermediates in many organic reactions. This idea, first proposed by
He dubbed the relationship between color and salt formation halochromy, of which malachite green is a prime example.
Carbocations are reactive intermediates in many organic reactions. This idea, first proposed by
 Alkyl-substituted carbocations follow the order in stability, as can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for , , , and ). The effect of alkyl substitution is a strong one: tertiary cations are stable and many are directly observable in
Alkyl-substituted carbocations follow the order in stability, as can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for , , , and ). The effect of alkyl substitution is a strong one: tertiary cations are stable and many are directly observable in  In terms of reactivity, carbocations are susceptible to attack by
In terms of reactivity, carbocations are susceptible to attack by 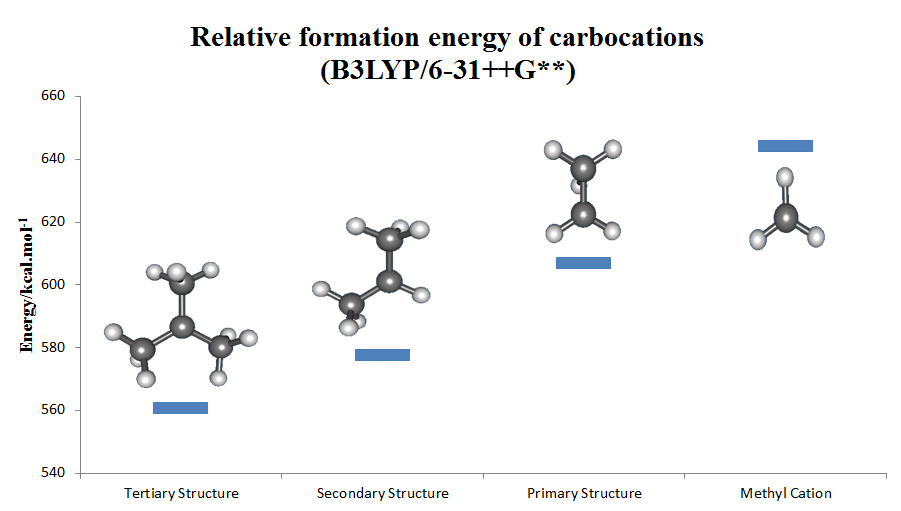 Carbocations typically undergo rearrangement reactions from less stable structures to equally stable or more stable ones by migration of an alkyl group or hydrogen to the cationic center to form a new carbocationic center. This often occurs with
Carbocations typically undergo rearrangement reactions from less stable structures to equally stable or more stable ones by migration of an alkyl group or hydrogen to the cationic center to form a new carbocationic center. This often occurs with  A carbocation may be stabilized by
A carbocation may be stabilized by  The effect of hyperconjugation is strongly stabilizing for carbocations: hyperconjugation with alkyl substituents is often as stabilizing or even more so than conjugation with a π system. Although conjugation to unsaturated groups results in significant stabilization by the mesomeric effect (resonance), the benefit is partially offset by the presence of a more electronegative sp2 or sp carbon next to the carbocationic center. Thus, as reflected by hydride ion affinities, a secondary carbocation is more stabilized than the allyl cation, while a tertiary carbocation is more stabilized than the benzyl cation — results that may seem counterintuitive on first glance.
Oxocarbenium and iminium ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the formal positive charge on an oxygen or nitrogen atom, respectively.
The effect of hyperconjugation is strongly stabilizing for carbocations: hyperconjugation with alkyl substituents is often as stabilizing or even more so than conjugation with a π system. Although conjugation to unsaturated groups results in significant stabilization by the mesomeric effect (resonance), the benefit is partially offset by the presence of a more electronegative sp2 or sp carbon next to the carbocationic center. Thus, as reflected by hydride ion affinities, a secondary carbocation is more stabilized than the allyl cation, while a tertiary carbocation is more stabilized than the benzyl cation — results that may seem counterintuitive on first glance.
Oxocarbenium and iminium ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the formal positive charge on an oxygen or nitrogen atom, respectively.
 Substituted cyclopropylcarbinyl cations have also been studied by NMR:
:
Substituted cyclopropylcarbinyl cations have also been studied by NMR:
: In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups, indicating that the
In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups, indicating that the  In terms of
In terms of
Press Release
The 1994 Nobel Prize in Chemistry". Nobelprize.org. 9 Jun 2010 {{Authority control Reactive intermediates

carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
. Among the simplest examples are the methenium , methanium
In chemistry, methanium is a complex positive ion with formula []+, namely a molecule with one carbon atom covalent bond, bonded to three hydrogen atoms and one hydrogen molecule, bearing a +1 electric charge. It is a superacid and one of the ...
and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ).
Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ion
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In older literature the name carbonium ion was used for this class, but now it refers exclusivel ...
s and five in the carbonium ion
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms.
The ...
s. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocation
In organic chemistry, the term 2-norbornyl cation (or 2-bicyclo .2.1eptyl cation) describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studie ...
s', which are carbocations that contain bridging C–C or C–H σ-bonds. However, others have more narrowly defined the term 'carbonium ion' as formally protonated or alkylated alkanes (, where R is H or alkyl), to the exclusion of non-classical carbocations like the 2-norbornyl cation.
Definitions
According to theIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
, a ''carbocation'' is any cation containing an even number of electrons in which a significant portion of the positive charge resides on a carbon atom. Prior to the observation of five-coordinate carbocations by Olah and coworkers, ''carbocation'' and ''carbonium ion'' were used interchangeably. Olah proposed a redefinition of ''carbonium ion'' as a carbocation featuring any type of three-center two-electron bonding, while a ''carbenium ion'' was newly coined to refer to a carbocation containing only two-center two-electron bonds with a three-coordinate positive carbon. Subsequently, others have used the term ''carbonium ion'' more narrowly to refer to species that are derived (at least formally) from electrophilic attack of H+ or R+ on an alkane, in analogy to other main group onium species, while a carbocation that contains any type of three-centered bonding is referred to as a ''non-classical carbocation''. In this usage, 2-norbornyl cation is not a carbonium ion, because it is formally derived from protonation of an alkene (norbornene) rather than an alkane, although it is a non-classical carbocation due to its bridged structure. The IUPAC acknowledges the three divergent definitions of carbonium ion and urges care in the usage of this term. For the remainder of this article, the term ''carbonium ion'' will be used in this latter restricted sense, while ''non-classical carbocation'' will be used to refer to any carbocation with C–C and/or C–H σ-bonds delocalized by bridging.
Since the late 1990s, most textbooks have stopped using the term carbonium ion for the classical three-coordinate carbocation. However, some university-level textbooks continue to use the term carbocation as if it were synonymous with carbenium ion, or discuss carbocations with only a fleeting reference to the older terminology of carbonium ions or carbenium and carbonium ions. One textbook retains the older name of carbonium ion for carbenium ion to this day, and uses the phrase ''hypervalent carbonium ion'' for .
A carbocation with a two-coordinate positive carbon derived from formal removal of a hydride ion (H−) from an alkene is known as a ''vinyl cation''. In the absence of geometric constraints, most substituted vinyl cations carry the formal positive charge on an sp-hydridized carbon atom of linear geometry. A two-coordinate approximately sp2-hybridized cation resulting from the formal removal of a hydride ion from an arene is termed an ''aryl cation''. These carbocations are relatively unstable (aryl cations especially so) and are infrequently encountered. Hence, they are frequently omitted from introductory and intermediate level textbooks. The IUPAC definition stipulates that carbocations are even-electron species; hence, radical cations like that are frequently encountered in mass spectrometry are not considered to be carbocations.
History
The history of carbocations dates back to 1891 when G. Merling reported that he added bromine to tropylidene ( cycloheptatriene) and then heated the product to obtain a crystalline, water-soluble material, . He did not suggest a structure for it; however, Doering and Knox convincingly showed that it wastropylium
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of ...
(cycloheptatrienylium) bromide. This ion is predicted to be aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
by Hückel's rule.
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol
Triphenylmethanol (also known as triphenylcarbinol, TrOH) is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions ...
gives deep-yellow solutions in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
. Triphenylmethyl chloride similarly formed orange complexes with aluminium and tin chlorides. In 1902, Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo and developed a nomenclature for cyclic compounds (that was subsequently extended and adopted as part of the IUPAC org ...
recognized the salt-like character of the compounds formed. Trityl carbocation (shown below) as a stable carbocationic system has been used as homogeneous organocatalyst in organic synthesis.
Julius Stieglitz
Julius Oscar Stieglitz (May 26, 1867 – January 10, 1937) was an American chemist of German Jewish origin. He was a teacher and organic chemist with a major interest in pharmaceutical and medicinal chemistry. He is known for the Stieglitz rearran ...
in 1899, was further developed by Hans Meerwein
Hans Meerwein (May 20, 1879 in Hamburg, Germany – October 24, 1965 in Marburg, Germany) was a German chemist.
Several reactions and reagents bear his name, most notably the Meerwein–Ponndorf–Verley reduction, the Wagner–Meerwein rearr ...
in his 1922 study of the Wagner–Meerwein rearrangement. Carbocations were also found to be involved in the SN1 reaction, the E1 reaction, and in rearrangement reactions such as the Whitmore 1,2 shift. The chemical establishment was reluctant to accept the notion of a carbocation and for a long time the Journal of the American Chemical Society refused articles that mentioned them.
The first NMR spectrum
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
of a stable carbocation in solution was published by Doering et al. in 1958. It was the heptamethylbenzenium
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.
For historic reasons this complex is also called a Wheland intermediate, after American chemist George ...
ion, made by treating hexamethylbenzene with methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industria ...
and aluminium chloride. The stable 7-norbornadienyl cation was prepared by Story et al. in 1960 by reacting norbornadienyl chloride with silver tetrafluoroborate in sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
at −80 °C. The NMR spectrum established that it was non-classically bridged (the first stable non-classical ion
Nonclassical carbocations are stabilized by charge delocalization from contributions of neighbouring or bonds, which can form bridged intermediates or transition states. Nonclassical ions have been extensively studied with the 2-norbornyl sy ...
observed).
In 1962, Olah directly observed the ''tert''-butyl carbocation by nuclear magnetic resonance as a stable species on dissolving ''tert''-butyl fluoride in magic acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960s ...
. The NMR of the norbornyl cation was first reported by Schleyer et al. and it was shown to undergo proton-scrambling over a barrier by Saunders et al.
Structure and properties
Carbonium ions can be thought of as protonated alkanes. Although alkanes are usually considered inert, under superacid conditions (e.g., HF/), the C-H sigma bond can act as a donor to . This results in a species that contains a 3c-2e bond between a carbon and two hydrogen atoms, a type of bonding common in boron chemistry, though relatively uncommon for carbon. As an alternative view point, the 3c-2e bond of carbonium ions could be considered as a molecule of coordinated to a carbenium ion (''see below''). Indeed, carbonium ions frequently decompose by loss of molecular hydrogen to form the corresponding carbenium ion. Structurally, the methanium ion is computed to have a minimum energy structure of ''C''s symmetry. However, the various possible structures of the ion are close in energy and separated by shallow barriers. Hence, the structure of the ion is often described asfluxional
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in s ...
. Although there appear to be five bonds to carbon in carbonium ions, they are not hypervalent, as the electron count around the central carbon is only eight, on account of the 3c-2e bond.
In contrast, at least in a formal sense, carbenium ions are derived from the protonation (addition of ) or alkylation (addition of ) of a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" m ...
or alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
. Thus, in at least one of their resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscil ...
depictions, they possess a carbon atom bearing a formal positive charge that is surrounded by a sextet of electrons (six valence electrons) instead of the usual octet required to fill the valence shell of carbon ( octet rule). Therefore, carbenium ions (and carbocations in general) are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge. In accord with VSEPR and Bent's rule, unless geometrically constrained to be pyramidal (e.g., 1-adamantyl cation), 3-coordinate carbenium ions are usually trigonal planar, with a pure p character empty orbital as its lowest unoccupied molecular orbital (LUMO) and CH/CC bonds formed from C(sp2) orbitals. A prototypical example is the methyl cation, . For the same reasons, carbocations that are 2-coordinate (vinyl cations) are generally linear in geometry, with CH/CC bonds formed from C(sp) orbitals.
 Alkyl-substituted carbocations follow the order in stability, as can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for , , , and ). The effect of alkyl substitution is a strong one: tertiary cations are stable and many are directly observable in
Alkyl-substituted carbocations follow the order in stability, as can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for , , , and ). The effect of alkyl substitution is a strong one: tertiary cations are stable and many are directly observable in superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
media, but secondary cations are usually transient and only the isopropyl, ''s''-butyl, and cyclopentyl cations have been observed in solution. There is seldom any experimental support for primary carbocations in the solution phase, even as transient intermediates (the ethyl cation has been proposed for reactions in 99.9% sulfuric acid and in ), and methyl cation has only been unambiguously identified in the gas phase. In most, if not all cases, the ground state of alleged primary carbocations consist of bridged structures in which positive charge is shared by two or more carbon atoms and are better described as side-protonated alkenes, edge-protonated cyclopropanes, or corner-protonated cyclopropanes rather than true primary cations. Even the simple ethyl cation, , has been demonstrated experimentally and computationally to be bridged and can be thought of as a symmetrically protonated ethylene molecule. The same is true for higher homologues like 1-propyl and 1-butyl cations. Neopentyl derivatives are thought to ionize with concomitant migration of a methyl group (anchimeric assistance
In organic chemistry, neighbouring group participation (NGP, also known as anchimeric assistance) has been defined by the International Union of Pure and Applied Chemistry (IUPAC) as the interaction of a reaction centre with a lone pair of elec ...
); thus, in most if not all cases, a discrete neopentyl cation is not believed to be involved.
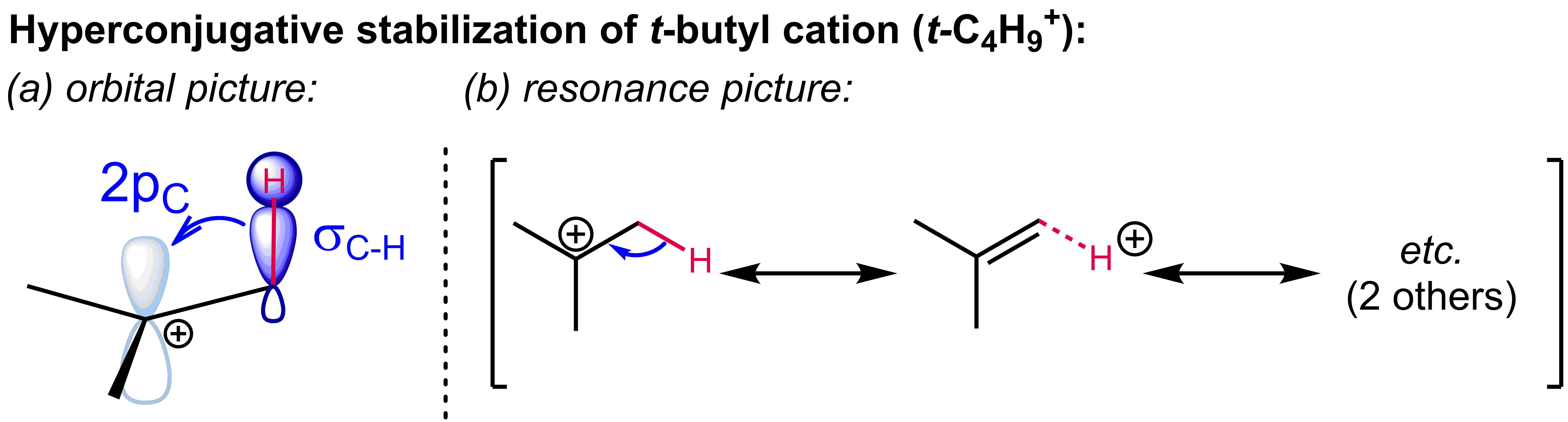
The stabilization by alkyl groups is explained by hyperconjugation. The donation of electron density from a β C-H or C-C bond into the unoccupied p orbital of the carbocation (a σCH/CC → p interaction) allows the positive charge to be delocalized.
Based on hydride ion affinity, the parent vinyl cation is less stable than even a primary sp2-hybridized carbocation, while an α alkyl-substituted vinyl cation has a stability that is comparable to the latter. Hence, vinyl cations are relatively uncommon intermediates. They can be generated by the ionization of a vinyl electrophile, provided the leaving group is sufficiently good (e.g., , IPh, or ). They have been implicated as intermediates in some vinyl substitution reactions (designated as SN1(vinyl)) and as intermediates in the electrophilic addition reactions of arylalkynes. With the exception of the parent vinyl cation, which is believed to be a bridged species, and geometrically constrained cyclic vinyl cations, most vinyl cations take on sp hybridization and are linear.
Aryl cations are more unstable than vinyl cations, due to the ring-enforced distortion to a nonlinear geometry and approximately sp2-character of the unoccupied orbital. Only in aryldiazonium salts is a good enough leaving group for the chemical generation of aryl cations.
Alkynyl cations are extremely unstable, much less stable than even (hydride ion affinity 386 kcal/mol versus 312 kcal/mol for ) and cannot be generated by purely chemical means. They can, however, be generated radiochemically via the beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
of tritium:
:
nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
s, like water, alcohols, carboxylates, azide, and halide ions, to form the addition product. Strongly basic nucleophiles, especially hindered ones, favor elimination over addition. Because even weak nucleophiles will react with carbocations, most can only be directly observed or isolated in non-nucleophilic media like superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
s.
 Carbocations typically undergo rearrangement reactions from less stable structures to equally stable or more stable ones by migration of an alkyl group or hydrogen to the cationic center to form a new carbocationic center. This often occurs with
Carbocations typically undergo rearrangement reactions from less stable structures to equally stable or more stable ones by migration of an alkyl group or hydrogen to the cationic center to form a new carbocationic center. This often occurs with rate constant In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction.
For a reaction between reactants A and B to form product C
the reaction rate is often found to have the ...
s in excess of 1010 s−1 at ambient temperature and still takes place rapidly (compared to the NMR timescale) at temperatures as low as −120 °C (''see Wagner-Meerwein shift''). In especially favorable cases like the 2-norbornyl cation, hydrogen shifts may still take place at rates fast enough to interfere with X-ray crystallography at . Typically, carbocations will rearrange to give a tertiary isomer. For instance, all isomers of rapidly rearrange to give the 1-methyl-1-cyclopentyl cation. This fact often complicates synthetic pathways. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce about one third 3-chloropentane and two thirds 2-chloropentane. The Friedel–Crafts alkylation suffers from this limitation; for this reason, the acylation (followed by Wolff–Kishner or Clemmensen reduction to give the alkylated product) is more frequently applied.
 A carbocation may be stabilized by
A carbocation may be stabilized by resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscil ...
by a carbon–carbon double bond or by the lone pair of a heteroatom adjacent to the ionized carbon. In order for a carbocation to be resonance-stabilized, the molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of find ...
of the donating group must have the proper symmetry, orientation, and energy level to interact with the empty 2p orbital of the carbocation. Such cations as '' allyl'' cation and '' benzyl'' cation are more stable than most other carbocations due to donation of electron density from π systems to the cationic center. Furthermore, carbocations present in aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
molecules are especially stabilized, largely due to the delocalized π electrons characteristic of aromatic rings. Molecules that can form allyl or benzyl carbocations are especially reactive. These carbocations where the is adjacent to another carbon atom that has a double or triple bond have extra stability because of the overlap of the empty p orbital of the carbocation with the p orbitals of the π bond. This overlap of the orbitals allows the positive charge to be dispersed and electron density from the π system to be shared with the electron-deficient center, resulting in stabilization. The doubly- and triply-benzylic carbocations, diphenylcarbenium and triphenylcarbenium
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethy ...
(trityl) cation, are particularly stable. For the same reasons, the partial p character of strained C–C bonds in cyclopropyl groups also allows for donation of electron density and stabilizes the ''cyclopropylmethyl'' (cyclopropylcarbinyl) cation.
The stability order of carbocations, from most stable to least stable as reflected by hydride ion affinity (HIA) values, are as follows (HIA values in kcal/mol in parentheses):
As noted in the history section, the tropylium cation () was one of the first carbocations to be discovered, due to its aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
stability. This carbocation is so stabilized that the molecule can be isolated and sold as a salt. On the other hand, the antiaromatic cyclopentadienyl cation () is destabilized by some 40 kcal/mol. The cyclopropenium cation (), although somewhat destabilized by angle strain, is still clearly stabilized by aromaticity when compared to its open-chain analog, allyl cation. These varying cation stabilities, depending on the number of π electrons in the ring system, can furthermore be crucial factors in reaction kinetics. The formation of an aromatic carbocation is much faster than the formation of an anti-aromatic or open-chain carbocation. Given the role of carbocations in many reaction schemes, such as SN1 for example, choosing the conjugation of starting materials can be a powerful method for conferring kinetic
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and ent ...
favorability or disfavorability, as the rate constant for any given step is dependent on the step's activation energy according to the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in ...
.
 The effect of hyperconjugation is strongly stabilizing for carbocations: hyperconjugation with alkyl substituents is often as stabilizing or even more so than conjugation with a π system. Although conjugation to unsaturated groups results in significant stabilization by the mesomeric effect (resonance), the benefit is partially offset by the presence of a more electronegative sp2 or sp carbon next to the carbocationic center. Thus, as reflected by hydride ion affinities, a secondary carbocation is more stabilized than the allyl cation, while a tertiary carbocation is more stabilized than the benzyl cation — results that may seem counterintuitive on first glance.
Oxocarbenium and iminium ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the formal positive charge on an oxygen or nitrogen atom, respectively.
The effect of hyperconjugation is strongly stabilizing for carbocations: hyperconjugation with alkyl substituents is often as stabilizing or even more so than conjugation with a π system. Although conjugation to unsaturated groups results in significant stabilization by the mesomeric effect (resonance), the benefit is partially offset by the presence of a more electronegative sp2 or sp carbon next to the carbocationic center. Thus, as reflected by hydride ion affinities, a secondary carbocation is more stabilized than the allyl cation, while a tertiary carbocation is more stabilized than the benzyl cation — results that may seem counterintuitive on first glance.
Oxocarbenium and iminium ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the formal positive charge on an oxygen or nitrogen atom, respectively.
Non-classical ions
Some carbocations such as the 2-norbornyl cation exhibit more or less symmetrical three-center two-electron bonding. Such structures, referred to asnon-classical carbocation
In organic chemistry, the term 2-norbornyl cation (or 2-bicyclo .2.1eptyl cation) describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studie ...
s, involve the delocalization of the bonds involved in the σ-framework of the molecule, resulting in C–C and C–H bonds of fractional bond order. This delocalization results in additional stabilization of the cation. For instance, depicted as a classical carbenium ion, 2-norbornyl cation appears to be a secondary carbocation. However, it is more stable than a typical "secondary" carbocation, being roughly as stable as a tertiary carbocation like ''t''-butyl cation, according to hydride ion affinity.
The existence of non-classical carbocations was once the subject of great controversy. On opposing sides were Herbert C. Brown
Herbert Charles Brown (May 22, 1912 – December 19, 2004) was an American chemist and recipient of the 1979 Nobel Prize in Chemistry for his work with organoboranes.
Life and career
Brown was born Herbert Brovarnik in London, to Ukrainian Jewis ...
, who believed that the what appeared to be a non-classical carbocation represents the average of two rapidly equilibrating classical species (or possibly two structures exhibiting some degree of bridging or leaning but is nevertheless not symmetric) and that the true non-classical structure is a transition state between the two potential energy minima, and Saul Winstein
Saul Winstein (October 8, 1912 – November 23, 1969) was a Jewish Canadian chemist who discovered the '' Winstein reaction.'' He argued a non-classical cation was needed to explain the stability of the norbornyl cation. This fueled a debat ...
, who believed that a non-classical structure that possessed a plane of symmetry was the sole potential energy minimum and that the classical structures merely two contributing resonance forms of this non-classical species. George Olah's discovery of superacidic media to allow carbocations to be directly observed, together with a very sensitive NMR technique developed by Martin Saunders to distinguish between the two scenarios, played important roles in resolving this controversy. At least for the 2-norbornyl cation itself, the controversy has been settled overwhelmingly in Winstein's favor, with no sign of the putative interconverting classical species, even at temperatures as low as 6 K, and a 2013 crystal structure showing a distinctly non-classical structure. A variety of carbocations (e.g., ethyl cation, ''see above'') are now believed to adopt non-classical structures. However, in many cases, the energy difference between the two possible "classical" structures and the "non-classical" one is very small, and it may be difficult to distinguish between the two possibilities experimentally.
Specific carbocations
A non-classical structure for is supported by substantial experimental evidence from solvolysis experiments and NMR studies conducted in non-nucleophilic media. One or both of two structures, the cyclopropylcarbinyl cation and the bicyclobutonium cation, were invoked to account for the observed reactivity in various experiments, while the NMR data point to a highly fluxional system that undergoes rapid rearrangement to give an averaged spectrum consisting of only two 13C NMR signals, even at temperatures as low as −132 °C. Computationally, it was confirmed that the energetic landscape of the system is very flat, and that the two isomers postulated based on experimental data are very close in energy, the bicyclobutonium structure being computed to be just 0.4 kcal/mol more stable than the cyclopropylcarbinyl structure. In the solution phase (SbF5·SO2ClF·SO2F2, with as the counterion), the bicyclobutonium structure predominates over the cyclopropylcarbinyl structure in a 84:16 ratio at −61 °C. Three other possible structures, two classical structures (the homoallyl cation and cyclobutyl cation) and a more highly delocalized non-classical structure (the tricyclobutonium ion), are now known to be less stable isomers (or merely a transition state rather than an energy minimum in the case of the cyclobutyl cation). Substituted cyclopropylcarbinyl cations have also been studied by NMR:
:
Substituted cyclopropylcarbinyl cations have also been studied by NMR:
:molecular conformation
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of a ...
of this cation is not perpendicular (as in A), which possesses a mirror plane, but is bisected (as in B) with the empty p-orbital parallel to the cyclopropyl ring system:
:bent bond
In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a ...
theory, this preference is explained by assuming favorable orbital overlap between the filled cyclopropane bent bonds and the empty p-orbital.
Pyramidal carbocation
See also
* Armilenium * Carbanion *Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" m ...
* ''Carbo''-mer
* Oxocarbenium
References
External links
*Press Release
The 1994 Nobel Prize in Chemistry". Nobelprize.org. 9 Jun 2010 {{Authority control Reactive intermediates