|
Phosphazene
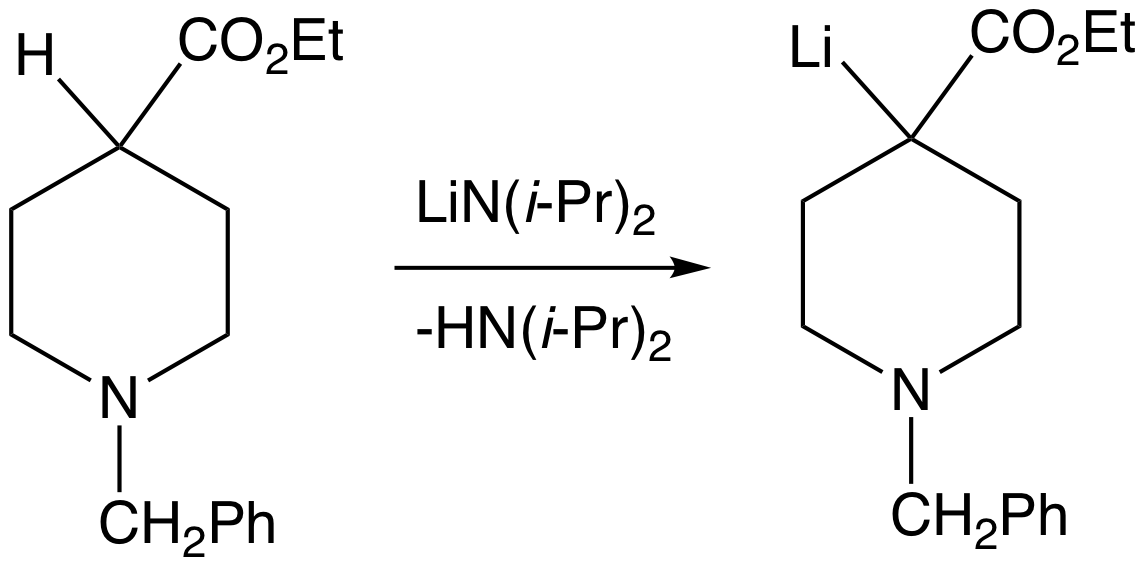
Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula . These phosphazenes are also known as iminophosphoranes and phosphine imides. They are superbases. Another class of compounds called phosphazenes are represented with the formula , where X = halogen, alkoxy group, amide and other organyl groups. One example is hexachlorocyclotriphosphazene . Bis(triphenylphosphine)iminium chloride is also referred to as a phosphazene, where Ph = phenyl group. This article focuses on those phosphazenes with the formula . Phosphazene bases Phosphazene bases are strong non-metallic non-ionic and low-nucleophilic bases. They are stronger bases than regular amine or amidine bases. Protonation takes place at a doubly bonded nitrogen atom. Related to phosphazene bases are the Verkade bases, which feature P(III) with three amido substituents and a transannular amine. The p''K''a's of , where R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P4-t-Bu
P4-''t''-Bu is a readily accessible chemical from the group of neutral, peralkylated sterically hindered polyaminophosphazenes, which are extremely strong bases but very weak nucleophiles, with the formula . "''t''-Bu" stands for ''tert''-butyl –. "P4" stands for the fact that this molecule has 4 phosphorus atoms. P4-''t''-Bu can also be regarded as tetrameric triaminoiminophosphorane of the basic structure . The homologous series of P1 to P7 polyaminophosphazenes of the general formula R^1_2N)_3P=N-\mathit-(R^1_2N)_P=NR2 with preferably methyl groups as R1, a methyl group or ''tert''-butyl group as and even-numbered ''x'' between 0 and 6 (P4-''t''-Bu: R1 = Me, R2 = ''t''-Bu and ''x'' = 3) has been developed by Reinhard Schwesinger; the resulting phosphazene bases are therefore also referred to as Schwesinger superbases. Preparation The convergent synthesis of P4-''t''-Bu is derived from phosphorus pentachloride (1) and leads in branch [] to the well-characterized aminotr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachlorocyclotriphosphazene
Hexachlorophosphazene is an inorganic compound with the formula . The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen centers, and can be viewed as a trimer of the hypothetical compound . Its classification as a phosphazene highlights its relationship to benzene. There is large academic interest in the compound relating to the phosphorus-nitrogen bonding and phosphorus reactivity. Occasionally, commercial or suggested practical applications have been reported, too, utilising hexachlorophosphazene as a precursor chemical.Mark, J. E.; Allcock, H. R.; West, R. “Inorganic Polymers” Prentice Hall, Englewood, NJ: 1992. . Derivatives of noted interest include the hexalkoxyphosphazene lubricants obtained from nucleophilic substitution of hexachlorophosphazene with alkoxides, or chemically resistant inorganic polymers with desirable thermal and mechanical properties known as polyphosphazenes produced from the polymerisation of hexachloropho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Imide
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR. While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides. Anions of this group, with the structure R3P=N−, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions. Synthesis Phosphine imides can be isolated as intermediates in the Staudinger reaction and have also been prepared by the action of hydroxylamine-O-sulfonic acid on phosphines, proceeding via a p-aminophosphonium salt. Reactions and applications The functional group will readily hydrolyse to give a phosphine oxide and an amine :R3P=NR' + H2O → R3P=O + R'NH2 Phosphinimide ligands of the general formula NPR3− form transition metal phosphinimide complexeses. Some of these complexes are potential catalysts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Verkade Base
In chemistry, a Verkade base (or Verkade superbase) is a superbase with the formula P(MeNCH2CH2)3N. A colorless oil, it is an aminophosphine although its inventor John Verkade called it proazaphosphatrane. The trimethyl derivative or 2,5,8,9-tetraza-1-phosphabicyclo .3.3ndecane is the simplest. Diverse analogues of the Verkade base are known, e.g. with isopropyl groups in place of methyl. Synthesis and reactions The Verkade base is generated by the reaction of N,N,N-trimethyltren with tris(dimethylamino)phosphine: :P(NMe2)3 + (MeNHCH2CH2)3N → P(MeNCH2CH2)3N + 3 Me2NH The principal reaction of the Verkade base is protonation. The proton is attacked by the Verkade base at phosphorus, which induces the formation of a transannular P-N bond. The product exemplifies the structure of an atrane. 290 px, left, Protonation of Verkade base. The conjugate acid P(MeNCH2CH2)3Nsup>+ has a pKa of 32.9 in acetonitrile. For comparison, the conjugate acid of triethylamine has a pKa n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(triphenylphosphine)iminium Chloride
Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula , often abbreviated , where Ph is phenyl , or even abbreviated PNl or NPl or PPNCl or PNPCl, where PPN or PNP stands for . This colorless salt is a source of the cation (abbreviated or ), which is used as an unreactive and weakly coordinating cation to isolate reactive anions. is a phosphazene. Synthesis and structure is prepared in two steps from triphenylphosphine : : This triphenylphosphine dichloride is related to phosphorus pentachloride . Treatment of this species with hydroxylamine in the presence of results in replacement of the two single P–Cl bonds in by one double P=N bond: : Triphenylphosphine oxide is a by-product. Bis(triphenylphosphine)iminium chloride is described as . The structure of the bis(triphenylphosphine)iminium cation is . The P=N=P angle in the cation is flexible, ranging from ~130 to 180° depending on the salt. Bent and linear forms of the P=N=P connections ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pivaldehyde
Pivaldehyde is an organic compound, more specifically an aldehyde. Shown in the image is a line-angle representation of this organic aldehyde, whose systematic name, 2,2-dimethylpropanal, is based on the longest carbon chain (three carbon atoms), ending in "-al" to indicate the aldehyde functionality, and where another descriptive synonym is trimethylacetaldehyde. Pivaldehyde is an example of an aldehyde with a sterically bulky R group, the ''tertiary''-butyl group (with 3 methyl groups, at lower left in the image), attached to the carbonyl, >C=O. By definition, the other "group", R', is a hydrogen (H) atom, shown here pointing directly upward. See also *Pivalic acid - corresponding carboxylic acid *Pivalamide - corresponding amide *Pinacolone Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion. On the other hand, a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a species formed by the removal of a proton from an acid, as in the reverse reaction it is able to gain a hydrogen ion. Because some acids are capable of releasing multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: :acid + base conjugate\ base + conjugate\ acid Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which proposed that any compound that can transfer a proton to any other compound is an acid, and the compound that accepts the proton is a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativity betwe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |