|
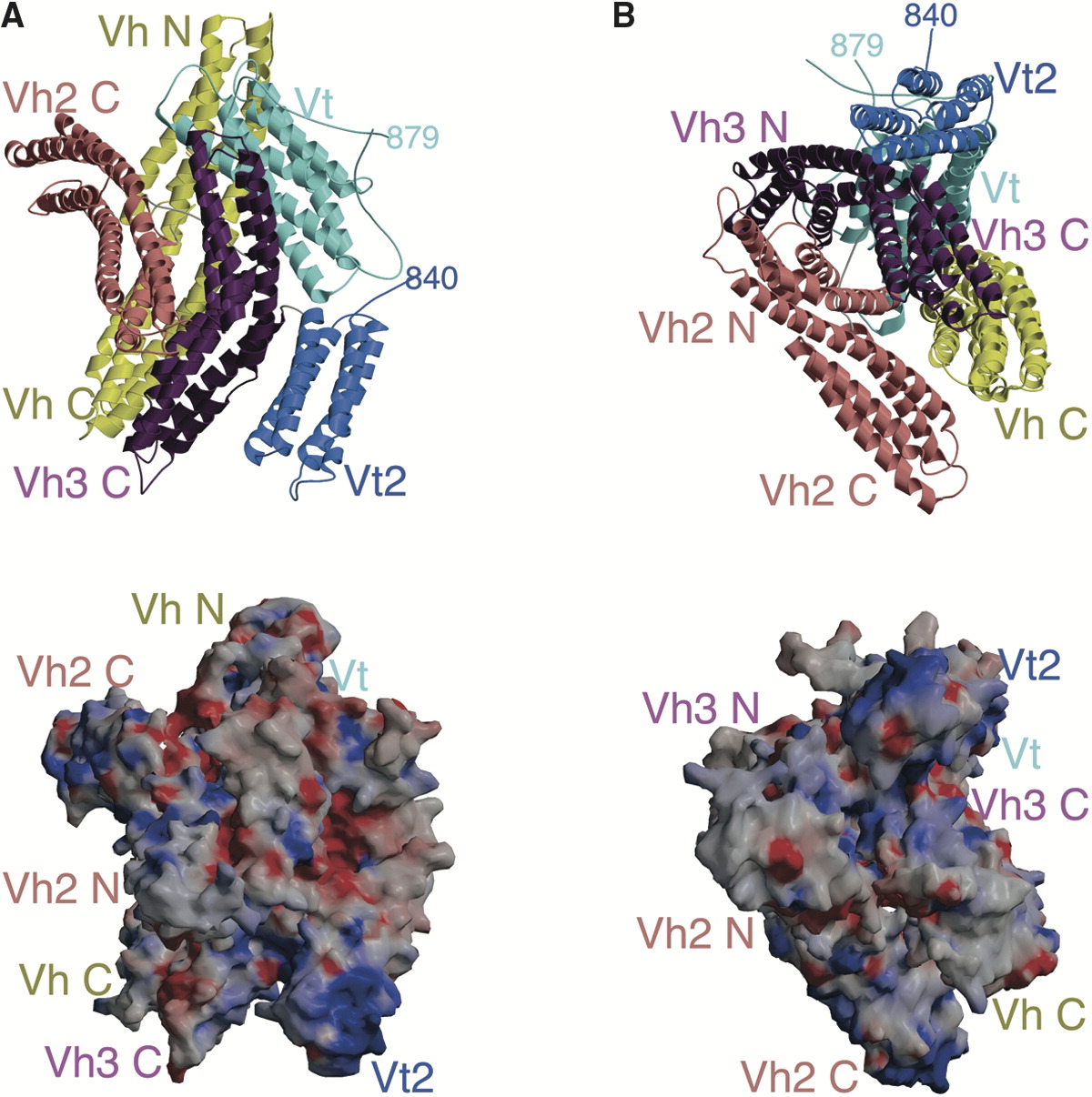
Paxillin
Paxillin is a protein that in humans is encoded by the ''PXN'' gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in ''PXN'' as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers. Structure Human paxillin is 64.5 kDa in molecular weight and 591 amino acids in length. The C-terminal region of paxillin is composed of four tandem double zinc finger LIM domains that are cysteine/histidine-rich with conserved repeats; these serve as binding sites for the protein tyrosine phosphatase-PEST, tubulin and serves as the targeting motif for focal adhesions. The N-terminal region of paxillin has five highly conserved leucine-rich sequences termed LD motifs, which mediate several interactions, including that with pp125FAK and vinculin. The LD motifs are predicted to form amphipathic alpha helices, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTK2
PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the ''PTK2'' gene. PTK2 is a focal adhesion-associated protein kinase involved in cellular adhesion (how cells stick to each other and their surroundings) and spreading processes (how cells move around). It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility. Function The PTK2 gene encodes a cytosolic protein tyrosine kinase that is found concentrated in the focal adhesions that form among cells attaching to extracellular matrix constituents. The encoded protein is a member of the FAK subfamily of protein tyrosine kinases that included PYK2, but lacks significant sequence similarity to kinases from other subfamilies. It also includes a large FERM domain. With the exception of certain types of blood cells, most cells express FAK. FAK tyrosine kinase activity can be activated, which plays a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinculin
In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane. Discovered independently by Benny Geiger and Keith Burridge, its sequence is 20%–30% similar to α- catenin, which serves a similar function. Binding alternately to talin or α-actinin, vinculin's shape and, as a consequence, its binding properties are changed. The vinculin gene occurs as a single copy and what appears to be no close relative to take over functions in its absence. Its splice variant metavinculin (see below) also needs vinculin to heterodimerize and work in a dependent fashion. Structure Vinculin is a 117-kDa cytoskeletal protein with 1066 amino acids. The protein contains an ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTPN12
Tyrosine-protein phosphatase non-receptor type 12 is an enzyme that in humans is encoded by the ''PTPN12'' gene. The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. This PTP contains a C-terminal PEST motif, which serves as a protein–protein interaction domain, and may be related to protein intracellular half-life. This PTP was found to bind and dephosphorylate the product of oncogene c-ABL, thus may play a role in oncogenesis. This PTP was shown to interact with, and dephosphorylate, various of cytoskeleton and cell adhesion molecules, such as p130 (Cas), CAKbeta/PTK2B, PSTPIP1, and paxillin, which suggested its regulatory roles in controlling cell shape and mobility. Interactions PTPN12 has been shown to interact with BCAR1, Grb2, PSTPIP1, TGFB1I1, Paxillin and SHC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paxilline
Paxilline is a toxic, tremorgenic diterpene indole polycyclic alkaloid molecule produced by ''Penicillium paxilli ''which was first characterized in 1975. Paxilline is one of a class of tremorigenic mycotoxins, is a potassium channel blocker, and is potentially genotoxic. Paxilline was found to significantly extend the lifespan, healthspan, and mobility of aged C. elegans worms, but had no such effect on young worms. Paxilline was not found to induce seizures when injected intracerebroventricularly in mice but paradoxically had anticonvulsant activity against picrotoxin and pentylenetetrazol seizures in mice. It has also been used in mice to induce autism-like behaviors through inhibition of the BK channel. Biosynthesis Paxiline biosynthesis starts with the synthesis of geranylgeranyl pyrophosphate via the terpenoid pathway and indole-3-glycerol phosphate, which is an intermediate in the tryptophan biosynthesis pathway. By expressing six genes known to be necessary for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keith Burridge
Keith Burridge (born 1 July 1950) is a British researcher and Kenan distinguished Professor at the University of North Carolina at Chapel Hill.ISI Highly Cited ResearcheAn essay by: Professor Keith Burridgein-cites.com His research on focal adhesions includes the discovery of many adhesion proteins including vinculin, talin and paxillin, and ranks him in top 1% of the most cited scientist in the field of molecular biology and genetics. Burridge has published more than 200 peer reviewed articles. Early life and education He was born in 1950 in Dorset, England. He obtained his undergraduate degree in 1971 from the University of Cambridge, and then completed his Ph.D. in Dennis Bray’s laboratory in the MRC Laboratory of Molecular Biology (LMB), also in Cambridge, in 1975. Using biochemical techniques, he showed that at least two distinct types of myosin II exist in non-muscle cells and that some cells expressed both types. Focal adhesion research He went as a postdoc to James D. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRKL
Crk-like protein is a protein that in humans is encoded by the CRKL gene. Function v-CRK avian sarcoma virus CT10-homolog-like contains one SH2 domain and two SH3 domains. CRKL has been shown to activate the RAS and JUN kinase signaling pathways and transform fibroblasts in a RAS-dependent fashion. It is a substrate of the BCR-ABL tyrosine kinase and plays a role in fibroblast transformation by BCR-ABL. In addition, CRKL has oncogenic potential. CrkL together with Crk participates in the Reelin signaling cascade downstream of DAB1. Interactions CRKL has been shown to interact Advocates for Informed Choice, doing business as, dba interACT or interACT Advocates for Intersex Youth, is a 501(c)(3) nonprofit organization using innovative strategies to advocate for the legal and human rights of children with intersex trai ... with: * Abl gene, * BCAR1, * BCR gene, * CBLB, * CD117, * CD34, * Cbl gene, * Dock2, * EPOR, * GAB1, * GAB2, * INPP5D, * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LIM Domain
LIM domains are protein structural domains, composed of two contiguous zinc fingers, separated by a two-amino acid residue hydrophobic linker. The domain name is an acronym of the three genes in which it was first identified (LIN-11, Isl-1 and MEC-3). LIM is a protein interaction domain that is involved in binding to many structurally and functionally diverse partners. The LIM domain appeared in eukaryotes sometime prior to the most recent common ancestor of plants, fungi, amoeba and animals. In animal cells, LIM domain-containing proteins often shuttle between the cell nucleus where they can regulate gene expression, and the cytoplasm where they are usually associated with actin cytoskeletal structures involved in connecting cells together and to the surrounding matrix, such as stress fibers, focal adhesions and adherens junctions. Discovery LIM domains are named after their initial discovery in the three homeobox proteins that have the following functions: * Lin-11 � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Focal Adhesions
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion. Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering. Structure and function Focal adhesions are integrin-containing, multi-protein structures that form mechanical links between intracellular actin bundles and the extracellular substrate in many cell types. Focal adhesions are large, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM. They are limited to clearly defined ranges of the cell, at which the pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Focal Adhesion
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion. Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering. Structure and function Focal adhesions are integrin-containing, multi-protein structures that form mechanical links between intracellular actin bundles and the extracellular substrate in many cell types. Focal adhesions are large, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM. They are limited to clearly defined ranges of the cell, at which the pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Finger
A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) in order to stabilize the fold. It was originally coined to describe the finger-like appearance of a hypothesized structure from the African clawed frog (''Xenopus laevis'') transcription factor IIIA. However, it has been found to encompass a wide variety of differing protein structures in eukaryotic cells. ''Xenopus laevis'' TFIIIA was originally demonstrated to contain zinc and require the metal for function in 1983, the first such reported zinc requirement for a gene regulatory protein followed soon thereafter by the Krüppel factor in ''Drosophila''. It often appears as a metal-binding domain in multi-domain proteins. Proteins that contain zinc fingers (zinc finger proteins) are classified into several different structural families. Unlike many other clearly defined supersecondary structures such as Greek keys or β hairpins, there are a number of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |