|
Pleuromutilin
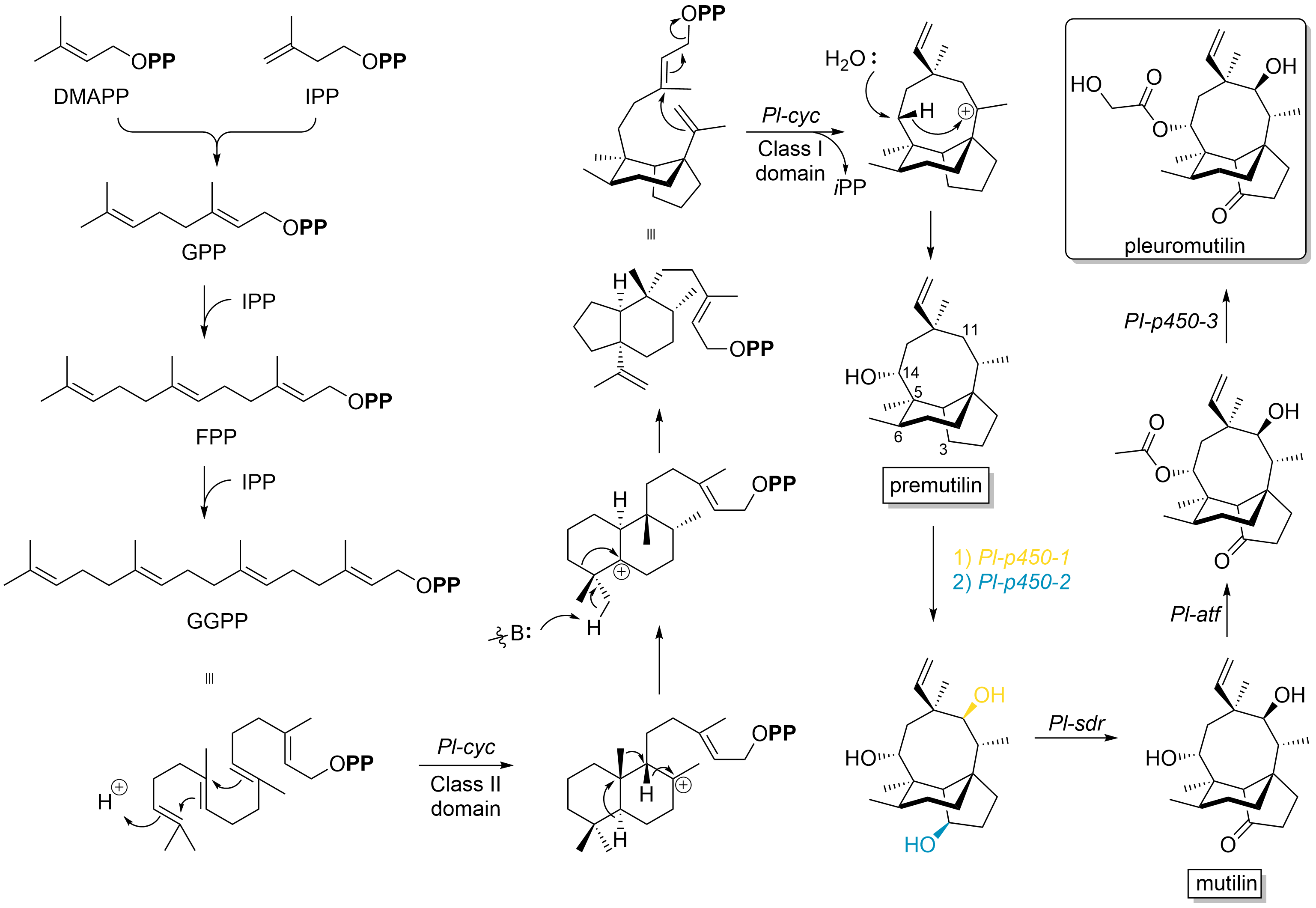
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi '' Omphalina mutila'' (formerly ''Pleurotus mutilus'') and '' Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in '' Drosophila subatrata'', '' Clitopilus scyphoides'', and some other '' Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate pathwa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleuromutilin Proposed Cyclisation Mechanism
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi '' Omphalina mutila'' (formerly ''Pleurotus mutilus'') and ''Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in '' Drosophila subatrata'', '' Clitopilus scyphoides'', and some other ''Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate pathway ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleuromutilin Antibiotics
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi '' Omphalina mutila'' (formerly ''Pleurotus mutilus'') and ''Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in '' Drosophila subatrata'', '' Clitopilus scyphoides'', and some other ''Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate pathway ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lefamulin
Lefamulin, sold under the brand name Xenleta, is an antibiotic medication used it to treat adults with community-acquired bacterial pneumonia. It is taken by mouth or by injection into a vein. Relatively common side effects include diarrhea, nausea, pain at the site of injection, and liver inflammation. It is a pleuromutilin antibiotic that inhibits the large subunit of bacterial ribosomes. Lefamulin was approved for medical use in the United States in August 2019, and in the European Union in July 2020. Medical uses Lefamulin is used to treat adults with community-acquired bacterial pneumonia. It was also investigated for treatment of acute bacterial skin and skin-structure infections (ABSSSI). Spectrum of activity Lefamulin has ''in vitro'' activity against ''Streptococcus pneumoniae'', '' viridans group Streptococci'', ''Moraxella catarrhalis'', ''Enterococcus faecium'', methicillin-resistant ''Staphylococcus aureus'' (MRSA), among other bacteria. History It was de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tiamulin
Tiamulin (previously thiamutilin) is a pleuromutilin antibiotic drug that is used in veterinary medicine particularly for pigs and poultry. Tiamulin is a diterpene Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being ... antimicrobial with a pleuromutilin chemical structure similar to that of valnemulin. References Pleuromutilin antibiotics Secondary alcohols Ketones Carboxylate esters Thioethers Cyclopentanes Diethylamino compounds Vinyl compounds {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutilin
Retapamulin is a topical antibiotic developed by GlaxoSmithKline. It is the first drug in the new class of pleuromutilin antibiotics to be approved for human use. It is marketed as an ointment under the brand names Altabax and Altargo. Retapamulin was approved by the United States Food and Drug Administration in April 2007 for the treatment of bacterial skin infections such as impetigo. In May 2007, retapamulin received approval in the EU from the European Medicines Agency for the same indication. Clinical trials have demonstrated its efficacy against certain Gram-positive bacteria including MRSA. Indications Retapamulin is indicated for the topical treatment of impetigo due to '' Staphylococcus aureus'' (methicillin-susceptible only) or ''Streptococcus pyogenes''. Pharmacology Mechanism of action Retapamulin is an antibacterial agent, specifically a protein synthesis inhibitor A protein synthesis inhibitor is a compound that stops or slows the growth or proliferation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retapamulin
Retapamulin is a topical antibiotic developed by GlaxoSmithKline. It is the first drug in the new class of pleuromutilin antibiotics to be approved for human use. It is marketed as an ointment under the brand names Altabax and Altargo. Retapamulin was approved by the United States Food and Drug Administration in April 2007 for the treatment of bacterial skin infections such as impetigo. In May 2007, retapamulin received approval in the EU from the European Medicines Agency for the same indication. Clinical trials have demonstrated its efficacy against certain Gram-positive bacteria including MRSA. Indications Retapamulin is indicated for the topical treatment of impetigo due to '' Staphylococcus aureus'' (methicillin-susceptible only) or ''Streptococcus pyogenes''. Pharmacology Mechanism of action Retapamulin is an antibacterial agent, specifically a protein synthesis inhibitor A protein synthesis inhibitor is a compound that stops or slows the growth or proliferation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valnemulin
Valnemulin (trade name Econor or Biotilina) is a pleuromutilin antibiotic used to treat swine dysentery, ileitis, colitis and pneumonia. It is also used for the prevention of intestinal The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans and ... infections of swine. Valnemulin has been observed to induce a rapid reduction of clinical symptoms of '' Mycoplasma bovis'' infection, and eliminate ''M. bovis'' from the lungs of calves. References Pleuromutilin antibiotics Secondary alcohols Ketones Thioethers Carboxamides Carboxylate esters Vinyl compounds {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azamulin
Azamulin is a pleuromutilin antibiotic. , it is not marketed in the US or Europe. In pharmacological studies, the substance is used as an inhibitor of the liver enzymes CYP3A4 and CYP3A5 Cytochrome P450 3A5 is a protein that in humans is encoded by the ''CYP3A5'' gene. Tissue distribution ''CYP3A5'' encodes a member of the cytochrome P450 superfamily of enzymes. Like most of the cytochrome P450, the CYP3A5 is expressed in the .... References CYP3A4 inhibitors Pleuromutilin antibiotics Experimental drugs {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidyl Transferase
The peptidyl transferase is an aminoacyltransferase () as well as the primary enzymatic function of the ribosome, which forms peptide bonds between adjacent amino acids using tRNAs during the translation process of protein biosynthesis. The substrates for the peptidyl transferase reaction are two tRNA molecules, one bearing the growing peptide chain and the other bearing the amino acid that will be added to the chain. The peptidyl chain and the amino acids are attached to their respective tRNAs via ester bonds to the O atom at the CCA-3' ends of these tRNAs. Peptidyl transferase is an enzyme that catalyzes the addition of an amino acid residue in order to grow the polypeptide chain in protein synthesis. It is located in the large ribosomal subunit, where it catalyzes the peptide bond formation. It is composed entirely of RNA. The alignment between the CCA ends of the ribosome-bound peptidyl tRNA and aminoacyl tRNA in the peptidyl transferase center contribute to its ability to cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Short-chain Dehydrogenase
The short-chain dehydrogenases/reductases family (SDR) is a very large family of enzymes, most of which are known to be NAD- or NADP-dependent oxidoreductases. As the first member of this family to be characterised was Drosophila alcohol dehydrogenase, this family used to be called 'insect-type', or 'short-chain' alcohol dehydrogenases. Most members of this family are proteins of about 250 to 300 amino acid residues. Most dehydrogenases possess at least 2 domains, the first binding the coenzyme, often NAD, and the second binding the substrate. This latter domain determines the substrate specificity and contains amino acids involved in catalysis. Little sequence similarity has been found in the coenzyme binding domain although there is a large degree of structural similarity, and it has therefore been suggested that the structure of dehydrogenases has arisen through gene fusion of a common ancestral coenzyme nucleotide sequence with various substrate specific domains. Subfamilies * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance (pharmacology), clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Ronald W. Estabrook, Estabrook, David Y. Cooper, Cooper, and Otto Rosenthal, Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of secondary metabolite, defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdom (biology), kingdoms of life: animals, plants, fungus, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |