|
Palaeoceanography
Paleoceanography is the study of the history of the oceans in the geologic past with regard to circulation, chemistry, biology, geology and patterns of sedimentation and biological productivity. Paleoceanographic studies using environment models and different proxies enable the scientific community to assess the role of the oceanic processes in the global climate by the re-construction of past climate at various intervals. Paleoceanographic research is also intimately tied to paleoclimatology. Source and methods of information Paleoceanography makes use of so-called proxy methods as a way to infer information about the past state and evolution of the world's oceans. Several geochemical proxy tools include long-chain organic molecules (e.g. alkenones), stable and radioactive isotopes, and trace metals. Additionally, sediment cores can also be useful; the field of paleoceanography is closely related to sedimentology and paleontology. Sea-surface temperature Sea-surface t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ocean
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth and contains 97% of Earth's water. An ocean can also refer to any of the large bodies of water into which the world ocean is conventionally divided."Ocean." ''Merriam-Webster.com Dictionary'', Merriam-Webster, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paleothermometer
A paleothermometer is a methodology that provides an estimate of the ambient temperature at the time of formation of a natural material. Most paleothermometers are based on empirically-calibrated proxy relationships, such as the tree ring or TEX86 methods. Isotope methods, such as the δ18O method or the clumped-isotope method, are able to provide, at least in theory, direct measurements of temperature. Common paleothermometers The isotopic ratio of 18O to 16O, usually in foram tests or ice cores. High values mean low temperatures. Confounded by ice volume - more ice means higher values. Ocean water is mostly H216O, with small amounts of HD16O and H218O. In Standard Mean Ocean Water (SMOW) the ratio of D to H is and 18O/16O is . Fractionation occurs during changes between condensed and vapour phases: the vapour pressure of heavier isotopes is lower, so vapour contains relatively more of the lighter isotopes and when the vapour condenses the precipitation preferentia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. It is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiocarbon
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through beta decay. A gram of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided. All known thorium isotopes are unstable. The most stable isotope, 232Th, has a half-life of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium are the only significantly radioactive elements that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sands as a by-product of extracti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protactinium
Protactinium (formerly protoactinium) is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel. The element was first identified in 1913 by Kazimierz Fajans and Oswald Helmuth Göhring and named ''brevium'' because of the short half-life of the specific isotope studied, i.e. protactinium-234. A more stable isotope of protactinium, 231Pa, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than the transition metals in groups 3 through 11. Cadmium and its congeners in group 12 are often not considered transition metals, in that they do not have partly filled ''d'' or ''f'' electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate. Cadmium occurs as a minor component in most zinc ores and is a byproduct of zinc production. Cadmium was used for a long time as a corrosion-resistant plating on steel, and cadmium compounds are used as red, orang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Cycle
The water cycle, also known as the hydrologic cycle or the hydrological cycle, is a biogeochemical cycle that describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice, fresh water, saline water (salt water) and atmospheric water is variable depending on a wide range of climatic variables. The water moves from one reservoir to another, such as from river to ocean, or from the ocean to the atmosphere, by the physical processes of evaporation, transpiration, condensation, precipitation, infiltration, surface runoff, and subsurface flow. In doing so, the water goes through different forms: liquid, solid (ice) and vapor. The ocean plays a key role in the water cycle as it is the source of 86% of global evaporation. The water cycle involves the exchange of energy, which leads to temperature changes. When water evaporates, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
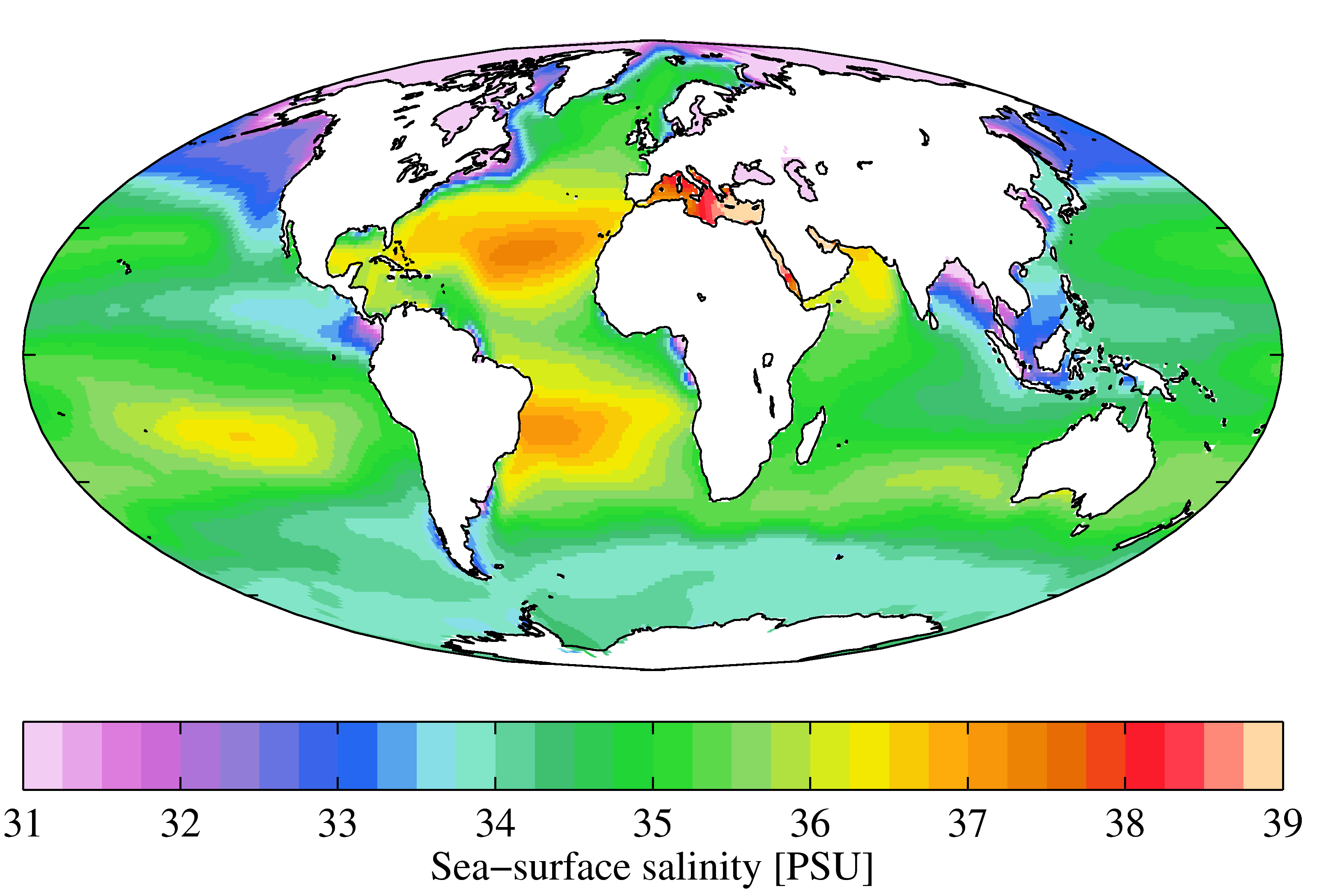
Salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |