|
Organoiron Compound
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "Green chemistry, greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but HSAB theory, hard ligands such as amines are employed as well. Iron(0) and more reduced states Carbonyl complexes Important metal carbonyls, iron carbonyls are the three neutral binary carbonyls, iron pentacarbonyl, diiron nonacarbonyl, and triiron d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Carbonyls
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes. Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of oxygen. Nomenclature and terminology The nomenclature of the metal carbonyls depends on the charge of the complex, the number and type of central atoms, and the number and type of ligands and their binding modes. They occur as neutral complexes, as positively-charged metal carbonyl cations or as negatively charged metal carbonylates. The carbon monoxide ligan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
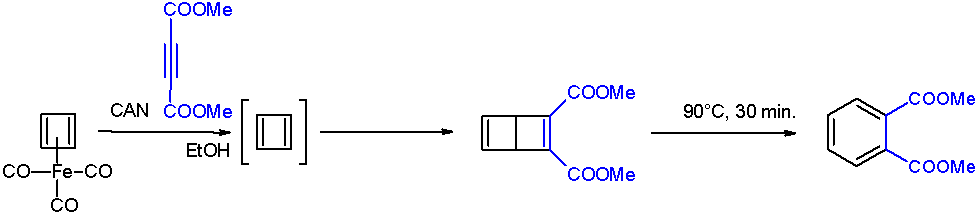
Cyclobutadieneiron Tricarbonyl
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive species in the free state. Preparation and structure It was first prepared in 1965 by Pettit from 3,4-dichlorocyclobutene and diiron nonacarbonyl: :C4H4Cl2 + 2 Fe2(CO)9 → (C4H4)Fe(CO)3 + 2 Fe(CO)5 + 5 CO + FeCl2 The compound is an example of a piano stool complex. The C-C distances are 1.426 Å. Properties Oxidative decomplexation of cyclobutadiene is achieved by treating the tricarbonyl complex with ceric ammonium nitrate. The released cyclobutadiene is trapped with a quinone, which functions as a dienophile. Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions, which can be classified as electrophilic aromatic substitution: : It undergoes Friedel-Crafts acylation with acetyl chloride and alu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protective Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctadiene
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH2)4(C2H2)2. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are the conjugated isomer 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition metal In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...s. These dienes are colorless volatile liquids.Thomas Schiffer, Georg Oenbrink “Cyclododecatriene, Cyclooctadiene, and 4-Vinylcyclohexene” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. References External links1,5-Cyclooctadiene Cycloalkenes Dienes Eight-membered rings {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbornadiene
Norbornadiene is an organic compound In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ... and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerization, isomerize (isomers would be anti-Bredt alkenes). Norbornadiene is also a useful Diels–Alder_reaction#The_dienophile, dienophile in Diels-Alder reactions. Synthesis Norbornadiene can be formed by a Diels-Alder reaction between cyclopentadiene and acetylene : Reactions Quadricyclane, a valence isomer, can be obtained from norbornadiene by a photochemical reaction when assisted by a photochemical sensitizer, sensiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexadiene
{{chemistry index ...
Cyclohexadiene may refer to: * 1,3-Cyclohexadiene, * 1,4-Cyclohexadiene, See also * Benzene or its theoretical isomer ''1,3,5-Cyclohexatriene'' * Cyclohexene Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |