|
Nodular Melanoma
Nodular melanoma (NM) is the most aggressive form of melanoma. It tends to grow more rapidly in thickness (vertically penetrate the skin) than in diameter compared to other melanoma subtypes. Instead of arising from a pre-existing mole, it may appear in a spot where a lesion did not previously exist. Since NM tends to grow in depth more quickly than it does in width, and can occur in a place that did not have a previous lesion, the prognosis is often worse because it takes longer for a person to be aware of the changes. NM is most often darkly pigmented; however, some NM lesions can be light brown, multicolored or even colorless (non-pigmented). A light-colored or non-pigmented NM lesion may escape detection because the appearance is not alarming, however an ulcerated and/or bleeding lesion is common. Polypoid melanoma is a virulent variant of nodular melanoma. The microscopic hallmarks are: * Dome-shaped at low power * Epidermis thin or normal * Dermal nodule of melanocytes with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye (uveal melanoma). In women, they most commonly occur on the legs, while in men, they most commonly occur on the back. About 25% of melanomas develop from moles. Changes in a mole that can indicate melanoma include an increase in size, irregular edges, change in color, itchiness, or skin breakdown. The primary cause of melanoma is ultraviolet light (UV) exposure in those with low levels of the skin pigment melanin. The UV light may be from the sun or other sources, such as tanning devices. Those with many moles, a history of affected family members, and poor immune function are at greater risk. A number of rare genetic conditions, such as xeroderma pigmentosum, also increase the risk. Diagnosis is by biopsy and analysis of any skin lesion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypoid Melanoma
Polypoid melanoma is a rare cutaneous condition, a virulent variant of nodular melanoma. Polypoid melanoma is a subtype of nodular melanoma, the most aggressive form of melanoma (a skin cancer). Polypoid melanoma, like all types of melanoma, starts in the cells that make melanin, which is the protective pigment that gives skin color. Polypoid melanoma is most commonly found on the torso but may be found in unexpected places like the nasal mucous membranes and the rectum. Sometimes polypoid melanoma may develop on moles on the skin, but it usually occurs out of nowhere on normal skin. Polypoid melanoma can be treated if it is diagnosed early, but the disease progresses very rapidly and has a worse prognosis than many other types of melanoma. Treatment Therapies for metastatic melanoma include the biologic immunotherapy agents ipilimumab, pembrolizumab, and nivolumab; BRAF inhibitors, such as vemurafenib and dabrafenib; and a MEK inhibitor trametinib. See also * Melanoma * List of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ipilimumab
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system. Cytotoxic T lymphocytes (CTLs) can recognize and destroy cancer cells. However, an inhibitory mechanism interrupts this destruction. Ipilimumab turns off this inhibitory mechanism and boosts the body's immune response against cancer cells. Ipilimumab was approved by the US Food and Drug Administration (FDA) in March 2011, for the treatment of melanoma, a type of skin cancer. It is undergoing clinical trials for the treatment of non-small cell lung carcinoma (NSCLC), small cell lung cancer (SCLC), (completed) bladder cancer (completed) and metastatic hormone-refractory prostate cancer. The concept of using anti-CTLA4 antibodies to treat cancer was first developed by James P. Allison while he was director of the Cancer Research Laboratory at the University of California, Berkeley. Cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pembrolizumab
Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is given by slow injection into a vein. Common side effects include fatigue, musculoskeletal pain, decreased appetite, itchy skin (pruritus), diarrhea, nausea, rash, fever (pyrexia), cough, difficulty breathing (dyspnea), constipation, pain, and abdominal pain. It is an IgG4 isotype antibody that blocks a protective mechanism of cancer cells and thereby, allows the immune system to destroy them. It targets the programmed cell death protein 1 (PD-1) receptor of lymphocytes. It works by targeting the cellular pathway of proteins found on the body's immune cells and some cancer cells, known as PD-1/PD-L1. Pembrolizumab was approved for medical use in the United States in 2014. In 2017, the US Food and Drug Administration (FDA) appr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nivolumab
Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein. The most common side effects include fatigue, rash, musculoskeletal pain, pruritus, diarrhea, nausea, asthenia, cough, dyspnea, constipation, decreased appetite, back pain, arthralgia, upper respiratory tract infection, pyrexia, headache, abdominal pain, and vomiting. Use during pregnancy may harm the baby and use when breastfeeding is not recommended. Nivolumab is a human IgG4 monoclonal antibody that blocks PD-1. It is a type of immunotherapy and works as a checkpoint inhibitor, blocking a signal that prevents activation of T cells from attackin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BRAF Inhibitor
BRAF is a human gene that encodes a protein called B-Raf. The gene is also referred to as proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B, while the protein is more formally known as serine/threonine-protein kinase B-Raf. The B-Raf protein is involved in sending signals inside cells which are involved in directing cell growth. In 2002, it was shown to be mutated in some human cancers. Certain other inherited ''BRAF'' mutations cause birth defects. Drugs that treat cancers driven by ''BRAF'' mutations have been developed. Two of these drugs, vemurafenib and dabrafenib are approved by FDA for treatment of late-stage melanoma. Vemurafenib was the first approved drug to come out of fragment-based drug discovery. Function B-Raf is a member of the Raf kinase family of growth signal transduction protein kinases. This protein plays a role in regulating the MAP kinase/ ERKs signaling pathway, which affects cell division, differentiation, and secretion. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vemurafenib
Vemurafenib (INN, marketed as Zelboraf) is an inhibitor of the B-Raf enzyme developed by Plexxikon (now part of Daiichi-Sankyo) and Genentech for the treatment of late-stage melanoma.; The name "vemurafenib" comes from V600E mutated BRAF inhibition. Approvals Vemurafenib received FDA approval for the treatment of late-stage melanoma on August 17, 2011, making it the first drug designed using fragment-based lead discovery to gain regulatory approval. Vemurafenib later received Health Canada approval on February 15, 2012. On February 20, 2012, the European Commission approved vemurafenib as a monotherapy for the treatment of adult patients with BRAF V600E mutation positive unresectable or metastatic melanoma, the most aggressive form of skin cancer. On November 6, 2017, the FDA approved Vemurafenib for the treatment of some patients with Erdheim–Chester disease (ECD), a rare type of histiocytic neoplasm. Mechanism of action Vemurafenib causes programmed cell death in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dabrafenib
Dabrafenib, sold under the brand name Tafinlar & Rafinlar ( both by Novartis) among others, is a medication for the treatment of cancers associated with a mutated version of the gene BRAF. Dabrafenib acts as an inhibitor of the associated enzyme B-Raf, which plays a role in the regulation of cell growth. Dabrafenib has clinical activity with a manageable safety profile in clinical trials of phase 1 and 2 in patients with BRAF (V600)-mutated metastatic melanoma. Approvals and indications The US Food and Drug Administration initially approved dabrafenib as a single agent treatment for patients with BRAF V600E mutation-positive advanced melanoma on May 29, 2013. Dabrafenib was approved for use in the European Union in August 2013. Clinical trial data demonstrated that resistance to dabrafenib and other BRAF inhibitors occurs within six to seven months. To overcome this resistance, the BRAF inhibitor dabrafenib was combined with the MEK inhibitor trametinib. On January 8, 2014, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEK Inhibitor
A MEK inhibitor is a chemical or drug that inhibits the mitogen-activated protein kinase kinase enzymes MEK1 and/or MEK2. They can be used to affect the MAPK/ERK pathway which is often overactive in some cancers. (See MAPK/ERK pathway#Clinical significance.) Hence MEK inhibitors have potential for treatment of some cancers, especially BRAF-mutated melanoma, and KRAS/BRAF mutated colorectal cancer. Approved for clinical use * Binimetinib (MEK162), approved by the FDA in June 2018 in combination with encorafenib for the treatment of patients with unresectable or metastatic BRAF V600E or V600K mutation-positive melanoma. * Cobimetinib or XL518, approved by US FDA in Nov 2015 for use in combination with vemurafenib (Zelboraf(R)), for treatment of advanced melanoma with a BRAF V600E or V600K mutation. * Selumetinib, had a phase 2 clinical trial for non-small cell lung cancer (NSCLC) which demonstrated an improvement in PFS, and is now in phase III development in KRAS mutation posit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trametinib
Trametinib, sold under the brand name Mekinist among others, is an anticancer medication used for the treatment of melanoma. It is a MEK inhibitor drug with anti-cancer activity. It inhibits MEK1 and MEK2. Trametinib had good results for metastatic melanoma carrying the BRAF V600E mutation in a phase III clinical trial. In this mutation, the amino acid valine (V) at position 600 within the BRAF protein has become replaced by glutamic acid (E) making the mutant BRAF protein constitutively active. In May 2013, trametinib was approved as a single-agent by the US Food and Drug Administration for the treatment of people with V600E mutated metastatic melanoma. Clinical trial data demonstrated that resistance to single-agent trametinib often occurs within 6 to 7 months. To overcome this, trametinib was combined with the BRAF inhibitor dabrafenib. As a result of this research, on January 8, 2014, the FDA approved the combination of dabrafenib and trametinib for the treatment of patie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
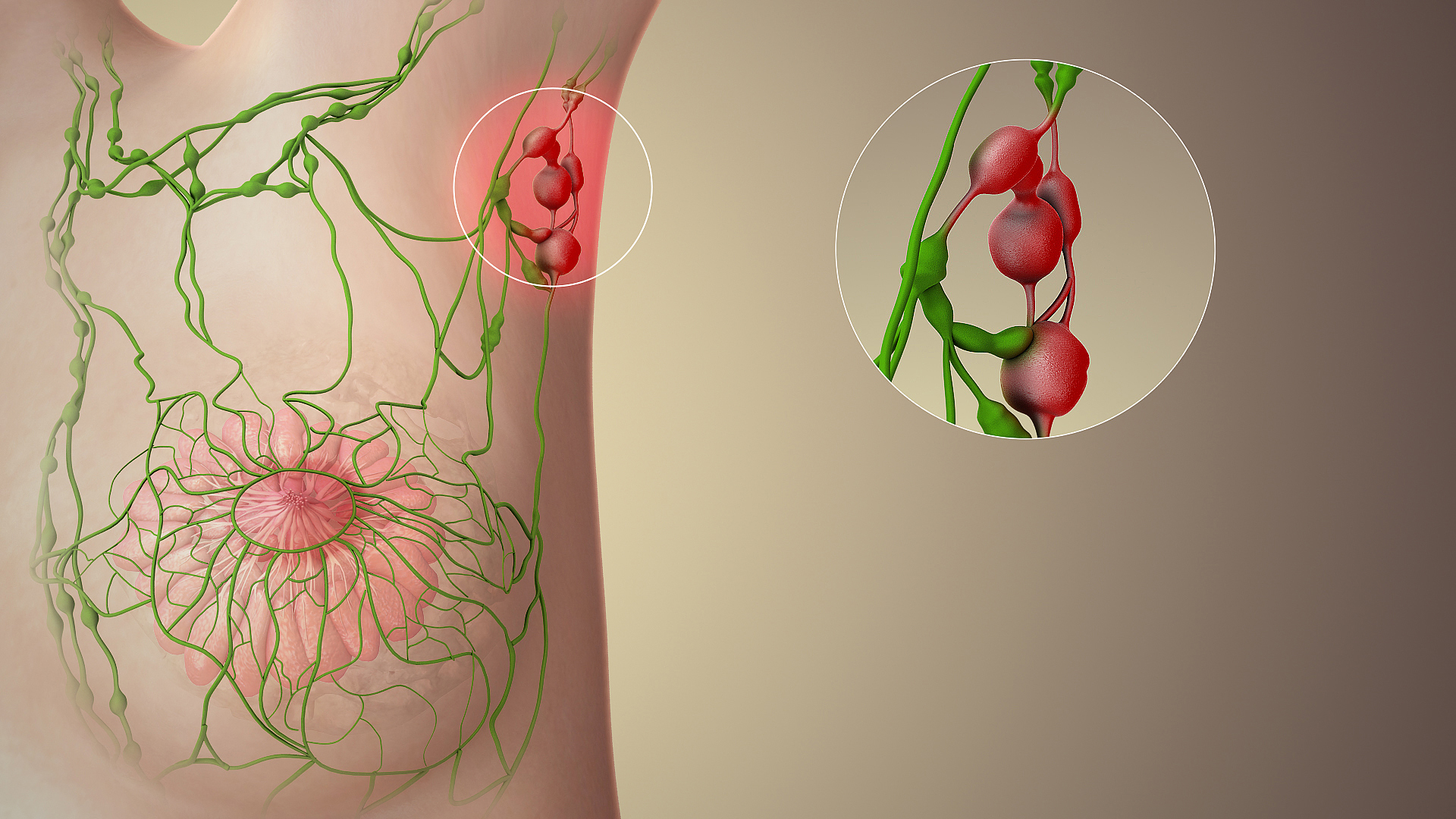
Sentinel Lymph Node
The sentinel lymph node is the hypothetical first lymph node or group of nodes draining a cancer. In case of established cancerous dissemination it is postulated that the sentinel lymph nodes are the target organs primarily reached by metastasizing cancer cells from the tumor. The sentinel node procedure (also termed sentinel lymph node biopsy or SLNB) is the identification, removal and analysis of the sentinel lymph nodes of a particular tumour. Physiology The spread of some forms of cancer usually follows an orderly progression, spreading first to regional lymph nodes, then the next echelon of lymph nodes, and so on, since the flow of lymph is directional, meaning that some cancers spread in a predictable fashion from where the cancer started. In these cases, if the cancer spreads it will spread first to lymph nodes (lymph glands) close to the tumor before it spreads to other parts of the body. The concept of sentinel lymph node surgery is to determine if the cancer has sprea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |