|
Naloxonazine
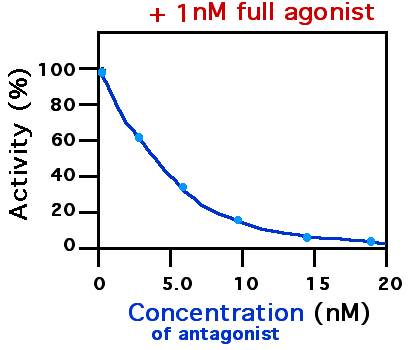
Naloxonazine is a potent, irreversible μ-opioid receptor The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(''mu'')-opioid peptide (MOP) receptors. The prototypical Π... antagonist. Naloxonazine forms spontaneously in acidic solutions of naloxazone, and may be responsible for much or all of the irreversible μ opioid receptor binding displayed by the latter. See also * Oxymorphone-3-methoxynaltrexonazine, a similar opioid also having two complete and mirrored morphinan carbon skeletons References 4,5-Epoxymorphinans Mu-opioid receptor antagonists Phenols Allyl compounds Semisynthetic opioids Alkylating agents Irreversible antagonists {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naloxazone
Naloxazone is an irreversible μ-opioid receptor antagonist which is selective for the μ1 receptor subtype. Naloxazone produces very long lasting antagonist effects as it forms a covalent bond to the active site of the μ-opioid receptor, thus making it impossible for the molecule to unbind and blocking the receptor permanently until the receptor is recycled by endocytosis. Naloxazone is the hydrazone analog of naloxone. It has been reported that naloxazone is unstable in acidic solution, dimerizing into the more stable and much more potent antagonist naloxonazine via the free NH2 of the hydrazone to form an azine linkage. Under conditions in which no naloxonazine formation could be detected, naloxazone did not display irreversible μ opioid receptor binding. See also * Chlornaltrexamine, an irreversible mixed agonist-antagonist * Oxymorphazone Oxymorphazone is an opioid analgesic drug related to oxymorphone. Oxymorphazone is a potent and long acting μ-opioid agonist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irreversible Antagonist
An irreversible antagonist is a type of antagonist that binds permanently to a receptor, either by forming a covalent bond to the active site, or alternatively just by binding so tightly that the rate of dissociation is effectively zero at relevant time scales. This permanently deactivates the receptor and is usually followed by rapid internalisation and recycling of the non-functional receptor protein. Irreversible enzyme inhibitors that act similarly are clinically used and include drugs such as aspirin, omeprazole and monoamine oxidase inhibitors.Rang and Dale's Pharmacology. (6th edition, 2007). p19. Examples * Naloxazone * Phenoxybenzamine See also * Irreversible agonist An irreversible agonist is a type of agonist that binds permanently to a receptor in such a manner that the receptor is permanently activated. It is distinct from a mere (reversible) agonist in that the association of an agonist to a receptor is ... * Irreversible enzyme inhibitor References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta blocker [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Life Sciences (journal)
''Life Sciences'' is a weekly peer-reviewed scientific journal covering research on the molecular, cellular, and physiological mechanisms of pharmacotherapy. It was started in 1962 by Pergamon Press. According to the ''Journal Citation Reports'', ''Life Sciences'' has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 6.780. The current Editor in Chief is Loren E. Wold. References External links * {{Authority control Pharmacology journals Elsevier academic journals Weekly journals Publications established in 1962 English-language journals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxymorphone-3-methoxynaltrexonazine
Oxymorphone-3-methoxynaltrexonazine (OM-3-MNZ) is a morphinan-based opioid that acts as a selective μ-opioid receptor agonist, unlike the closely related mixed agonist-antagonist Oxymorphonenaltrexonazine. See also * Naloxonazine * Naloxone * Naltrexone * Oxymorphone Oxymorphone (sold under the brand names Numorphan and Opana among others) is a highly potent opioid analgesic indicated for treatment of severe pain. Pain relief after injection begins after about 5–10 minutes, after oral administration it beg ... References Cyclopropanes 4,5-Epoxymorphinans Opioids Phenol ethers {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Compounds
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semisynthetic Opioids
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, and suppressing cough. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal. Opioids can cause death and have been used for executions in the United States. Side effects of opioids may include itchiness, sedation, nausea, respiratory depression, constipation, and euphoria. Long-term use can cause tolerance, meaning that increased doses are required to achieve the same effect, and physical dependence, meaning that abruptly discontinuing the drug leads to unpleasant withdrawal symptoms. The euphoria attracts recreational use, and frequent, escalating recreational use o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylating Agents
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion (carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent bond b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |