|
Methanium
In chemistry, methanium is a complex positive ion with formula []+, namely a molecule with one carbon atom covalent bond, bonded to three hydrogen atoms and one hydrogen molecule, bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion. It is highly unstable and highly reactive even upon having a complete octet, thus granting its superacidic properties. Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids. It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova. It occurs as an intermediate species in chemical reactions. The methanium ion is named after methane (), by analogy with the derivation of ammonium ion () from ammonia (). Structure Fluxional methanium can be visualised as a carbenium ion with a molecule of hydrogen interacting with the empty orbital in a 3-center-2-electron bond. The bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonium Ion
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms. The next simplest carbonium ions after methanium have two carbon atoms. Ethynium, or protonated acetylene , and ethenium are usually classified in other families. The ethanium ion has been studied as an extremely rarefied gas by infrared spectroscopy. The isomers of octonium (protonated octane, ) have been studied. The carbonium ion has a planar geometry. In older literature, the name "carbonium ion" was used for what is today called carbenium. The current definitions were proposed by the chemist George Andrew Olah in 1972 and are now widely accepted. A stable carbonium ion is the complex pentakis(triphenylphosphinegold(I))methanium , produced by Schmidbauer and others. Preparation Carbonium ions can be obtained by treating alkanes w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onium Ion
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the namesake for the class, is ammonium, , the protonated derivative of ammonia, . The name onium is also used for cations that would result from the substitution of hydrogen atoms in those ions by other groups, such as organic radicals, or halogens; such as tetraphenylphosphonium, . The substituent groups may be divalent or trivalent, yielding ions such as iminium and nitrilium. A simple onium ion has a charge of +1. A larger ion that has two onium ion subgroups is called a double onium ion, and has a charge of +2. A triple onium ion has a charge of +3, and so on. Compounds of an onium cation and some other anion are known as onium compounds or onium salts. Onium ions and onium compounds are inversely analogous to ions and ate complexes: * Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methenium
In organic chemistry, methenium (also called methylium, carbenium, methyl cation, or protonated methylene) is a cation with the formula . It can be viewed as a methylene radical (:) with an added proton (), or as a methyl radical (•) with one electron removed. It is a carbocation and an enium ion, making it the simplest of the carbenium ions. Structure Experiments and calculations generally agree that the methenium ion is planar, with threefold symmetry. The carbon atom is a prototypical (and exact) example of sp2 hybridization. Preparation and reactions For mass spectrometry studies at low pressure, methenium can be obtained by ultraviolet photoionization of methyl radical, or by collisions of monatomic cations such as and with neutral methane. In such conditions, it will react with acetonitrile to form the ion . Upon capture of a low-energy electron (less than ), it will spontaneously dissociate. It is seldom encountered as an intermediate in the condensed phase. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluxional Molecule
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods. Spectroscopic studies Many organometallic compounds exhibit fluxionality. Fluxionality is however pervasive. NMR spectroscopy Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates compara ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Victor Talrose
Victor Lvovich Talrose (russian: Виктор Львович Тальрозе, April 15, 1922 – June 22, 2004) was a Russian scientist and mass spectrometrist. He was known as the “Father of Russian Mass Spectrometry” and was the author of 440 articles and 6 monographs. Early education Victor Talrose was born in Tula, Russia, an industrial city on the Upa River 200 kilometers (120 mi) south of Moscow. He was the son of a medical doctor. He graduated from high school in 1939 with a gold medal for outstanding abilities. He started his studies in chemistry at Moscow State University, but his studies were interrupted by the outbreak of World War II. Talrose served in the Soviet Army and was wounded and hospitalized three times. He returned to his studies in 1945, graduating in 1947. Radical reactions He began work on his master's degree in the Laboratory of Elementary Processes headed by Viktor Kondrat’ev. His thesis was on the role of the hydroperoxyl radical in gas pha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
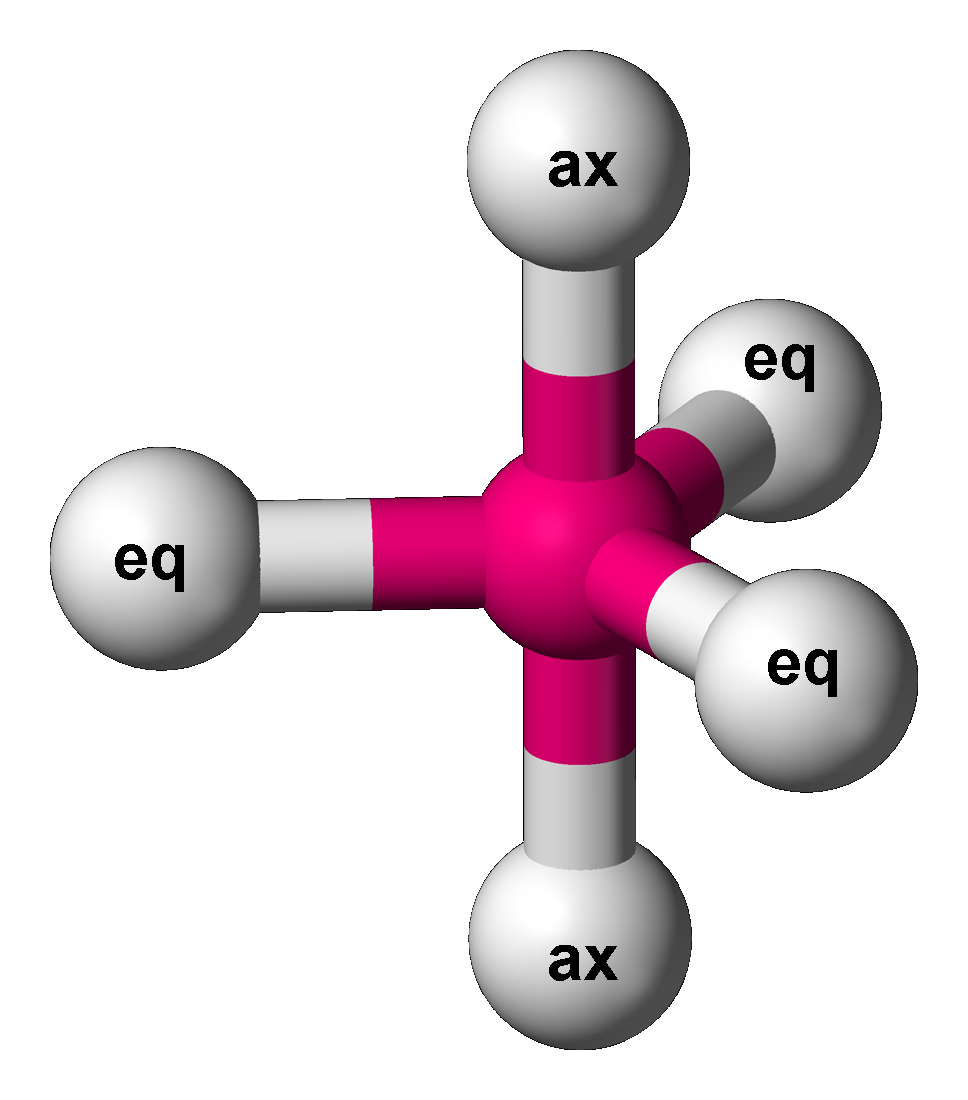
Berry Mechanism
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry Richard Stephen Berry (April 9, 1931 – July 26, 2020) was an American professor of physical chemistry. He was the James Franck Distinguished Service Professor Emeritus at The University of Chicago. He was also Special Advisor for National Sec ..., who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-center-2-electron Bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation () and diborane (). In these two structures, the three atoms in each 3c-2e bond form an angular geometry, leading to a bent bond. Boranes and carboranes An extended version of the 3c–2e bond model features heavily in cluster compounds described by the polyhedral skeletal electron pair theory, such as boranes and carboranes. These molecules derive their stability from having a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Fluoroantimonic acid is corrosive. For example, it cannot be contained directly in glass carboys, as it attacks glass, but can be stored in containers lined with PTFE (Teflon). Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of "fluoroantimonic acid" is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, – (such as ). Thus, the formula "" is a convenient but oversimplifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |