|
Methane Hydrates
Methane clathrate (CH4·5.75H2O) or (8CH4·46H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice. Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth. Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans. Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep sedimentary structures and form outcrops on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallisation of methane migrating from deep along geologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burning Hydrate Inlay US Office Naval Research
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While the activation energy must be overcome to initiate combustion (e.g., using a lit match to light a fire), the heat from a flame may provide enough energy to make the reaction self-sustaining. Combustion is often a complicated sequence of elementary radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot enough that incandescent light in the form of either glowing or a flame is produced. A simp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Core
An ice core is a core sample that is typically removed from an ice sheet or a high mountain glacier. Since the ice forms from the incremental buildup of annual layers of snow, lower layers are older than upper ones, and an ice core contains ice formed over a range of years. Cores are drilled with hand augers (for shallow holes) or powered drills; they can reach depths of over two miles (3.2 km), and contain ice up to 800,000 years old. The physical properties of the ice and of material trapped in it can be used to reconstruct the climate over the age range of the core. The proportions of different oxygen and hydrogen isotopes provide information about ancient temperatures, and the air trapped in tiny bubbles can be analysed to determine the level of atmospheric gases such as carbon dioxide. Since heat flow in a large ice sheet is very slow, the borehole temperature is another indicator of temperature in the past. These data can be combined to find the climate m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Number (chemistry)
The concept of magic numbers in the field of chemistry refers to a specific property (such as stability) for only certain representatives among a distribution of structures. It was first recognized by inspecting the intensity of mass-spectrometric signals of rare gas cluster ions. In case a gas condenses into clusters of atoms, the number of atoms in these clusters that are most likely to form varies between a few and hundreds. However, there are peaks at specific cluster sizes, deviating from a pure statistical distribution. Therefore, it was concluded that clusters of these specific numbers of rare gas atoms dominate due to their exceptional stability. The concept was also successfully applied to explain the monodispersed occurrence of thiolate-protected gold clusters; here the outstanding stability of specific cluster sizes is connected with their respective electronic configuration. The term magic numbers is also used in the field of nuclear physics. In this context, ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira ( ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/ MEDLINE/ PubMed, and the Science Citation Index Expanded. According to the '' Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydration Number
The hydration number, or solvation number of a compound is defined as the average number of molecules bound to the compound more strongly (by 13.3 kcal/mol or more) than they are bound to other water molecules. The hydration number is dependent on the concentration of the compound in solution, and the identity of the compound. When compounds are dissolved in water, the water molecules form a solvation shell surrounding the solute. For charged species, the orientation of water molecules around the solute is dependent on its ionic charge, with cations attracting water’s electronegative oxygen and anions attracting the hydrogens. Uncharged compounds such as methane can also be solvated by water and also have a hydration number. Although solvation shells can contain inner and outer shell solvent-solute interactions, the hydration number generally focuses on the inner shell solvent molecules that most directly interact with the solute. Background Given the overwhelming abundance of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetradecahedron
240px, A tetradecahedron with ''D2d'' symmetry, existing in the Weaire–Phelan structure A tetradecahedron is a polyhedron with 14 faces. There are numerous topologically distinct forms of a tetradecahedron, with many constructible entirely with regular polygon faces. A tetradecahedron is sometimes called a tetrakaidecahedron. No difference in meaning is ascribed. The Greek word '' kai'' means 'and'. There is evidence that mammalian epidermal cells are shaped like flattened tetrakaidecahedra, an idea first suggested by Lord Kelvin. The polyhedron can also be found in soap bubbles and in sintered ceramics, due to its ability to tesselate in 3D space. Convex There are 1,496,225,352 topologically distinct ''convex'' tetradecahedra, excluding mirror images, having at least 9 vertices. (Two polyhedra are "topologically distinct" if they have intrinsically different arrangements of faces and vertices, such that it is impossible to distort one into the other simply by changing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dodecahedron
In geometry, a dodecahedron (Greek , from ''dōdeka'' "twelve" + ''hédra'' "base", "seat" or "face") or duodecahedron is any polyhedron with twelve flat faces. The most familiar dodecahedron is the regular dodecahedron with regular pentagons as faces, which is a Platonic solid. There are also three regular star dodecahedra, which are constructed as stellations of the convex form. All of these have icosahedral symmetry, order 120. Some dodecahedra have the same combinatorial structure as the regular dodecahedron (in terms of the graph formed by its vertices and edges), but their pentagonal faces are not regular: The pyritohedron, a common crystal form in pyrite, has pyritohedral symmetry, while the tetartoid has tetrahedral symmetry. The rhombic dodecahedron can be seen as a limiting case of the pyritohedron, and it has octahedral symmetry. The elongated dodecahedron and trapezo-rhombic dodecahedron variations, along with the rhombic dodecahedra, are space-filling. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Properties Of Water
Water () is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. It is also the third most abundant molecule in the universe (behind molecular hydrogen and carbon monoxide). Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 °C for its molar mass, and a high heat capacity. Water is amphoteric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons ( protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
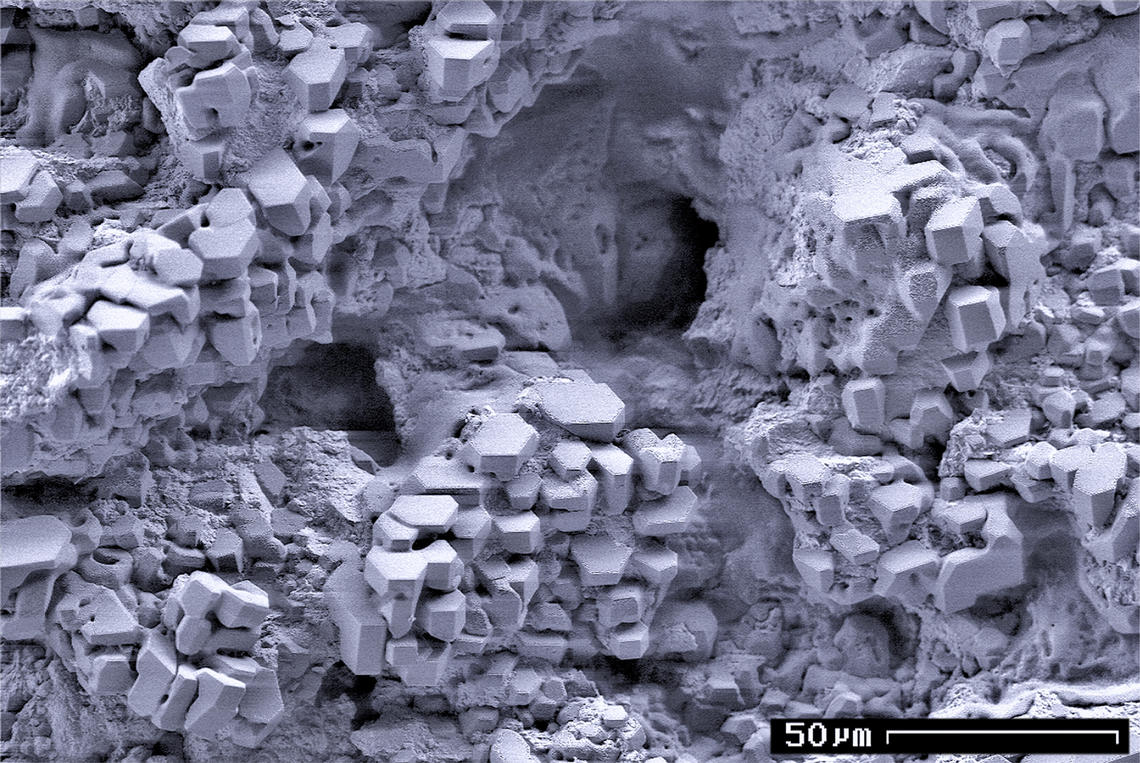
Gas Hydrate Crystals
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture, such as air, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colourless gas invisible to the human observer. The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |