|
Magic Number (chemistry)
The concept of magic numbers in the field of chemistry refers to a specific property (such as stability) for only certain representatives among a distribution of structures. It was first recognized by inspecting the intensity of mass spectrometry, mass-spectrometric signals of rare gas cluster ions. In case a gas condenses into Cluster (physics), clusters of atoms, the number of atoms in these clusters that are most likely to form varies between a few and hundreds. However, there are peaks at specific cluster sizes, deviating from a pure statistical distribution. Therefore, it was concluded that clusters of these specific numbers of rare gas atoms dominate due to their exceptional stability. The concept was also successfully applied to explain the monodispersed occurrence of Thiolate-protected gold cluster, thiolate-protected gold clusters; here the outstanding stability of specific cluster sizes is connected with their respective electronic configuration. The term magic number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated accordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster (physics)
In physics, the term clusters denotes small, polyatomic particles. As a rule of thumb, any particle made of between 3×100 and 3×107 atoms is considered a cluster. The term can also refer to the organization of protons and neutrons within an atomic nucleus, e.g. the alpha particle (also known as "α-cluster"), consisting of two protons and two neutrons (as in a helium nucleus). Overview Although first reports of cluster species date back to the 1940s, cluster science emerged as a separate direction of research in the 1980s, One purpose of the research was to study the gradual development of collective phenomena which characterize a bulk solid. For example, these are the color of a body, its electrical conductivity, its ability to absorb or reflect light, and magnetic phenomena such as ferro-, ferri-, or antiferromagnetism. These are typical collective phenomena which only develop in an aggregate of a large number of atoms. It was found that collective phenomena break down for v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
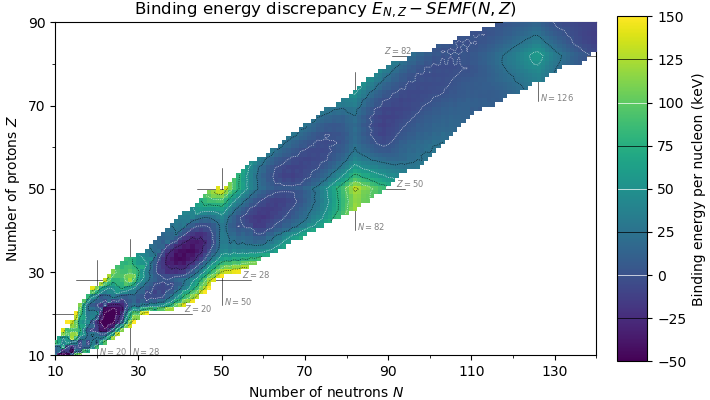
Magic Number (physics)
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126 . For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay. The unusual stability of isotopes having magic numbers means that transuranium elements could theoretically be created with extremely large nuclei and yet not be subject to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons. Discoveries in nuclear physics have led to applications in many fields. This includes nuclear power, nuclear weapons, nuclear medicine and magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. Such applications are studied in the field of nuclear engineering. Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the origin of the chemical elements. History The history of nuclear physics as a discipl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first", and this name was given to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutrons
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element that di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Shell Model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model is a model of the atomic nucleus which uses the Pauli exclusion principle to describe the structure of the nucleus in terms of energy levels. The first shell model was proposed by Dmitri Ivanenko (together with E. Gapon) in 1932. The model was developed in 1949 following independent work by several physicists, most notably Eugene Paul Wigner, Maria Goeppert Mayer and J. Hans D. Jensen, who shared the 1963 Nobel Prize in Physics for their contributions. The nuclear shell model is partly analogous to the atomic shell model, which describes the arrangement of electrons in an atom in that filled shell results in better stability. When adding nucleons (protons or neutrons) to a nucleus, there are certain points where the binding energy of the next nucleon is significantly less than the last one. This observation that there are specific magic quantum numbers of nucleons (2, 8, 20, 28, 50, 82, 126) wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Number (physics)
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126 . For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay. The unusual stability of isotopes having magic numbers means that transuranium elements could theoretically be created with extremely large nuclei and yet not be subject to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)