|
Mercury(II) Telluride
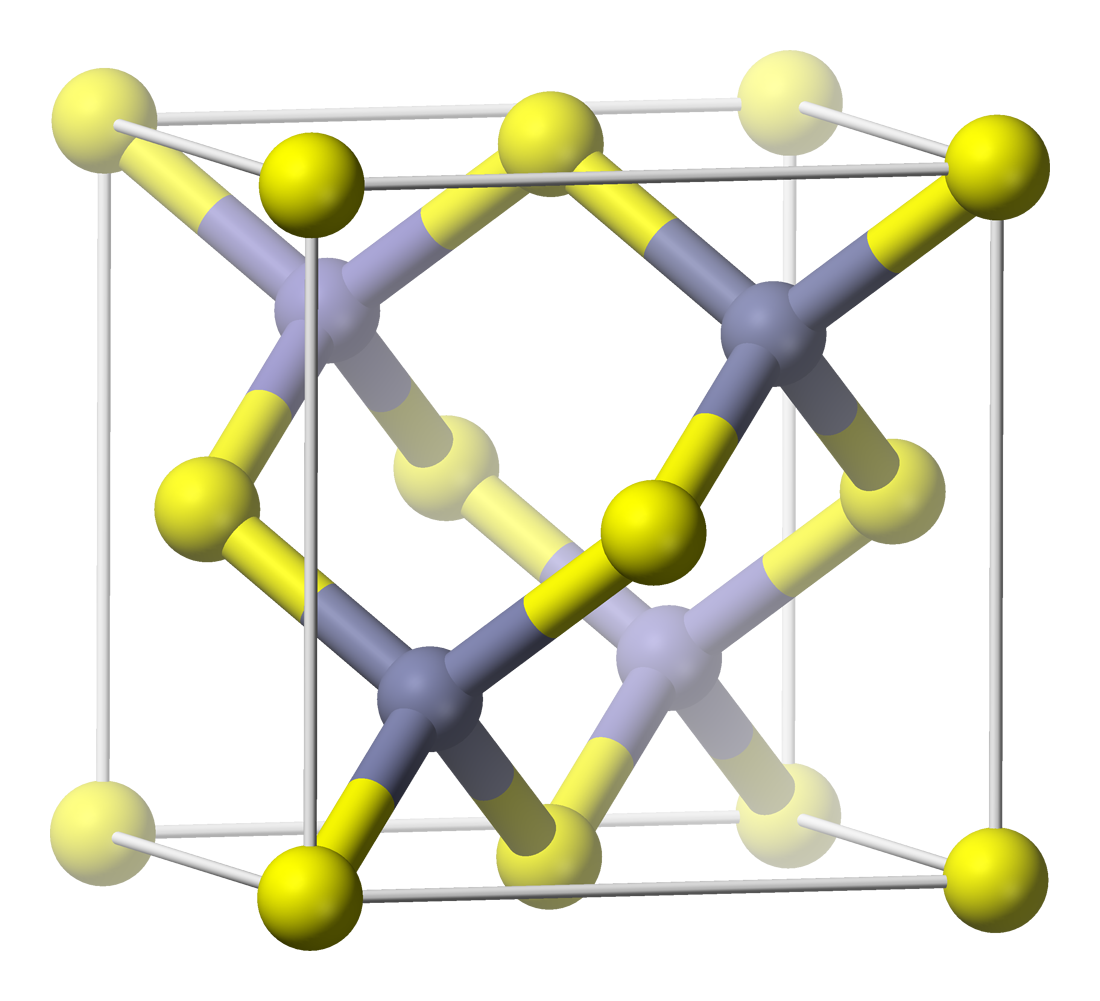
Mercury telluride (HgTe) is a binary chemical compound of mercury and tellurium. It is a semi-metal related to the II-VI group of semiconductor materials. Alternative names are mercuric telluride and mercury(II) telluride. HgTe occurs in nature as the mineral form coloradoite. Physical properties All properties are at standard temperature and pressure unless stated otherwise. The lattice parameter is about 0.646 nm in the cubic crystalline form. The bulk modulus is about 42.1 GPa. The thermal expansion coefficient is about 5.2×10−6/K. Static dielectric constant 20.8, dynamic dielectric constant 15.1. Thermal conductivity is low at 2.7 W·m2/(m·K). HgTe bonds are weak leading to low hardness values. Hardness 2.7×107 kg/m2. Doping N-type doping can be achieved with elements such as boron, aluminium, gallium, or indium. Iodine and iron will also dope n-type. HgTe is naturally p-type due to mercury vacancies. P-type doping is also achieved by introducing zinc, copper, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hosted lead-zinc ore deposits, Mississippi-Valley type, and Volcanogenic massive sulfide ore deposit, volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite (and other sulfide mineral, sulfides), calcite, dolomite (mineral), dolomite, quartz, rhodochrosite, and fluorite. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847, naming it based on the Greek word ''sphaleros'', meaning "deceiving", due to the difficulty of identifying the mineral. In addition to zinc, sphalerite is an ore of cadmium, gallium, germanium, and indium. Miners have been known to refer to sphalerite as ''zinc blende'', ''black-jack'', and ''ruby blende''. Marmatite is an opaque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Cadmium Telluride
Hg1−xCdxTe or mercury cadmium telluride (also cadmium mercury telluride, MCT, MerCad Telluride, MerCadTel, MerCaT or CMT) is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning the shortwave infrared to the very long wave infrared regions. The amount of cadmium (Cd) in the alloy can be chosen so as to tune the optical absorption of the material to the desired infrared wavelength. CdTe is a semiconductor with a bandgap of approximately 1.5 electronvolts (eV) at room temperature. HgTe is a semimetal, which means that its bandgap energy is zero. Mixing these two substances allows one to obtain any bandgap between 0 and 1.5 eV. Properties Physical Hg1−xCdxTe has a zincblende structure with two interpenetrating face-centered cubic lattices offset by (1/4,1/4,1/4)ao in the primitive cell. The cations Cd and Hg are statistically mixed on the yellow sublattice while the Te anions form the grey sublattice in the image ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Selenide
Mercury selenide (HgSe; sometimes mercury(II) selenide) is a chemical compound of mercury and selenium. It is a grey-black crystalline solid semi-metal with a sphalerite structure. The lattice constant is 0.608 nm. HgSe occurs naturally as the mineral Tiemannite, and is a component of the "intimate mixture" of HgSe and Se known as HgSe2. Along with other II-VI compounds, colloidal nanocrystals of HgSe can be formed. Applications * Selenium is used in filters in some steel plants to remove mercury from exhaust gases. The solid product formed is HgSe. * HgSe can be used as an ohmic contact to wide-gap II-VI semiconductors such as zinc selenide or zinc oxide. Toxicity HgSe is non-toxic as long as it is not ingested due to its insolubility. Toxic hydrogen selenide fumes can be evolved on exposure to acids. HgSe is a relatively stable compound which might mean that it is less toxic than elemental mercury or many organometallic mercury compounds. Selenium's ability to comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Telluride
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadmium sulfide to form a p–n junction solar PV cell. Applications CdTe is used to make thin film solar cells, accounting for about 8% of all solar cells installed in 2011. They are among the lowest-cost types of solar cell, although a comparison of total installed cost depends on installation size and many other factors, and has changed rapidly from year to year. The CdTe solar cell market is dominated by First Solar. In 2011, around 2 GWp of CdTe solar cells were produced; For more details and discussion see cadmium telluride photovoltaics. CdTe can be alloyed with mercury to make a versatile infrared detector material (HgCdTe). CdTe alloyed with a small amount of zinc makes an excellent solid-state X-ray and gamma ray detector ( Cd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalorganic Vapour Phase Epitaxy
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation (later Rockwell International) Science Center by Harold M. Manasevit. Basic principles In MOCVD ultrapure precursor gases are injected into a reactor, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrobromic Acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid has a p''K''a of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known. Uses Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. HBr almost uniquely participates in anti-Markovnikov hydrohalogenation of alkenes. The resulting 1-bro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Formation
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it. In the International System of Units (SI), the unit of measurement for enthalpy is the joule. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Carrier
In physics, a charge carrier is a particle or quasiparticle that is free to move, carrying an electric charge, especially the particles that carry electric charges in electrical conductors. Examples are electrons, ions and holes. The term is used most commonly in solid state physics. In a conducting medium, an electric field can exert force on these free particles, causing a net motion of the particles through the medium; this is what constitutes an electric current. In conducting media, particles serve to carry charge: *In many metals, the charge carriers are electrons. One or two of the valence electrons from each atom are able to move about freely within the crystal structure of the metal. The free electrons are referred to as conduction electrons, and the cloud of free electrons is called a Fermi gas. Many metals have electron and hole bands. In some, the majority carriers are holes. *In electrolytes, such as salt water, the charge carriers are ions, which are atoms or molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface State
Surface states are electronic states found at the surface of materials. They are formed due to the sharp transition from solid material that ends with a surface and are found only at the atom layers closest to the surface. The termination of a material with a surface leads to a change of the electronic band structure from the bulk material to the vacuum. In the weakened potential at the surface, new electronic states can be formed, so called surface states. Origin at condensed matter interfaces As stated by Bloch's theorem, eigenstates of the single-electron Schrödinger equation with a perfectly periodic potential, a crystal, are Bloch waves : \begin \Psi_ &=\mathrm^u_(\boldsymbol). \end Here u_(\boldsymbol) is a function with the same periodicity as the crystal, ''n'' is the band index and k is the wave number. The allowed wave numbers for a given potential are found by applying the usual Born–von Karman cyclic boundary conditions. The termination of a crystal, i.e. the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or holes. In an electrolyte the charge carriers are ions, while in plasma, an ionized gas, they are ions and electrons. The SI unit of electric current is the ampere, or ''amp'', which is the flow of electric charge across a surface at the rate of one coulomb per second. The ampere (symbol: A) is an SI base unit. Electric current is measured using a device called an ammeter. Electric currents create magnetic fields, which are used in motors, generators, inductors, and transformers. In ordinary con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Topological Insulator
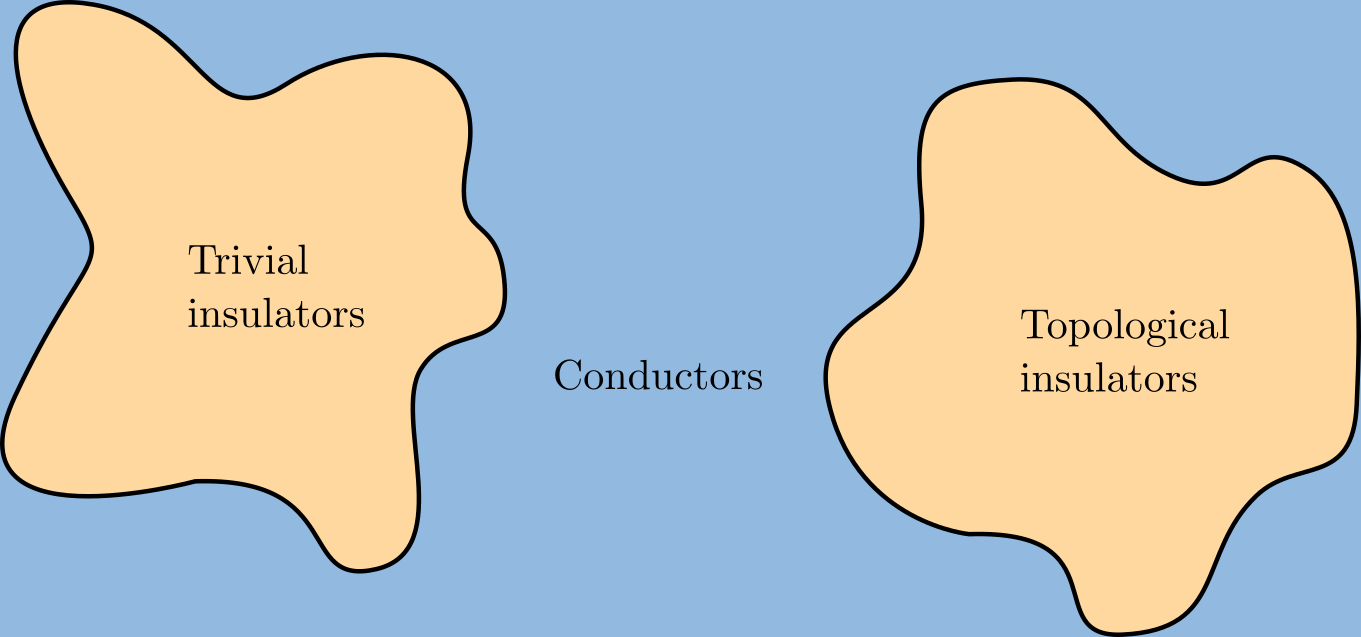
A topological insulator is a material whose interior behaves as an electrical insulator while its surface behaves as an electrical conductor, meaning that electrons can only move along the surface of the material. A topological insulator is an insulator for the same reason a "trivial" (ordinary) insulator is: there exists an energy gap between the valence and conduction bands of the material. But in a topological insulator, these bands are, in an informal sense, "twisted", relative to a trivial insulator. The topological insulator cannot be continuously transformed into a trivial one without untwisting the bands, which closes the band gap and creates a conducting state. Thus, due to the continuity of the underlying field, the border of a topological insulator with a trivial insulator (including vacuum, which is topologically trivial) is forced to support a conducting state. Since this results from a global property of the topological insulator's band structure, local (symmetry- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |