|
Mechanochemistry
Mechanochemistry (or mechanical chemistry) is the initiation of chemical reactions by mechanical phenomena. Mechanochemistry thus represents a fourth way to cause chemical reactions, complementing thermal reactions in fluids, photochemistry, and electrochemistry. Conventionally mechanochemistry focuses on the transformations of covalent bonds by mechanical force. Not covered by the topic are many phenomena: phase transitions, dynamics of biomolecules (docking, folding), and sonochemistry. Mechanochemistry also encompasses mechanophores which are molecules that undergo predictable changes in response to applied stress. Two types of mechanophores are mechanochromic ones in which a force causes a change in molecular structure and subsequently color and acid releasing mechanophores that release small amounts of an acid such as HCl in response to an applied force. Mechanochemistry is not the same as mechanosynthesis, which refers specifically to the machine-controlled construction of c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanosynthesis
Mechanosynthesis is a term for hypothetical chemical syntheses in which reaction outcomes are determined by the use of mechanical constraints to direct reactive molecules to specific molecular sites. There are presently no non-biological chemical syntheses which achieve this aim. Some atomic placement has been achieved with scanning tunnelling microscopes. Introduction In conventional chemical synthesis or chemosynthesis, reactive molecules encounter one another through random thermal motion in a liquid or vapor. In a hypothesized process of mechanosynthesis, reactive molecules would be attached to molecular mechanical systems, and their encounters would result from mechanical motions bringing them together in planned sequences, positions, and orientations. It is envisioned that mechanosynthesis would avoid unwanted reactions by keeping potential reactants apart, and would strongly favor desired reactions by holding reactants together in optimal orientations for many molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
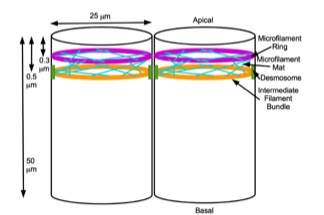
Embryonic Differentiation Waves
A mechanochemistry, mechanochemical based model for Neurulation#Primary neural induction, primary neural induction was first proposed in 1985 by Brodland and Richard Gordon (theoretical biologist), Gordon. They proposed that there is a mechanically sensitive bistable organelle made of microtubules and microfilaments in the apical ends of cells within cell sheets that are about to differentiate (that are competent) and these cells are under mechanical tension. The microtubules and microfilaments are in mechanical opposition in a proposed embryonic organelle they called the cell state splitter. Depending on where the cell is within a sheet, the tension will be resolved by either the apical end contracting or the apical end expanding. The resolution will begin at one point and spread over the rest of the tissue limited by other mechanical forces at boundaries. An actual physical wave of contraction has been found which traverses the presumptive neural epithelium of the developing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoparticles
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead. Nanoparticles are distinguished from microparticles (1-1000 μm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and ultrafast optical effects or electric properties. Being more subject to the Brownian motion, they usually do not sediment, like colloid, colloidal particles that conversely are usually understood to range from 1 to 1000 nm. Being much smaller than the wavelengths of visible light (400-700 nm), nanoparticles cannot be seen with ordinary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanoluminescence
Mechanoluminescence is light emission resulting from any mechanical action on a solid. It can be produced through ultrasound, or through other means. * Electrochemiluminescence is the emission induced by an electrochemical stimulus. * Fractoluminescence is caused by stress that results in the formation of fractures, that in turn yield light. * Piezoluminescence is caused by pressure that results in elastic deformation and large polarization from the piezoelectric effect. * Sonoluminescence is the emission of short bursts of light from imploding bubbles in a liquid when excited by sound. * Triboluminescence is nominally caused by rubbing, but sometimes occurs because of resulting fractoluminescence. It is often used as a synonym. See also *List of light sources This is a list of sources of light, the visible part of the electromagnetic spectrum. Light sources produce photons from another energy source, such as heat, chemical reactions, or conversion of mass or a different freque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
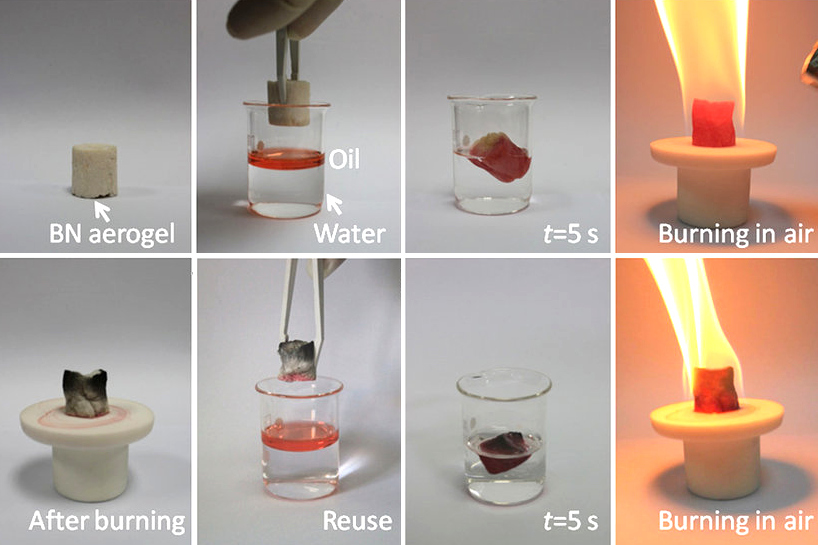
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. History Boron nitride was discovered by chemistry teacher of the Liverpool Institute in 1842 via reduction of boric acid with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, distinguished by the position and conformation of the double bond. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodate
An iodate is the polyatomic anion with the formula . It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid. Structure Iodate is pyramidal in structure. The O–I–O angles range from 97° to 105°, somewhat smaller than the O–Cl–O angles in chlorate. Reactions Redox Iodate is one of several oxyanions of iodine, and has an oxidation number of +5. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite: : Iodate oxidizes iodide in acidic conditions: : Similarly, chlorate oxidizes iodide to iodate: : Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide. Acid-base Iodate is unusual in that it forms a strong hydrogen bond with its parent ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphyne
Graphyne is an allotrope of carbon. Although it has been studied in theoretical models, it is very difficult to synthesize and only small amounts of uncertain purity have been created. Its structure is one-atom-thick Planar crystallographic group, planar sheets of sp and Sp2 bond, sp2-bonded carbon atoms arranged in crystal lattice. It can be seen as a lattice of benzene rings connected by acetylene bonds. The material is called graphyne-''n'' when benzene rings are connected by ''n'' sequential acetylene molecules, and graphdiyne for a particular case of ''n'' = 2 (diacetylene links). Depending on the content of acetylene groups, graphyne can be considered a mixed hybridization, sp''k'', where k can be 1 or 2, and thus differs from the hybridization of graphene (considered pure sp2) and diamond (pure sp3). First-principles calculations showed that periodic graphyne structures and their boron nitride analogues are stable. The calculations used Phonon scattering, phonon dispers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnets
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets. A permanent magnet is an object made from a material that is magnetized and creates its own persistent magnetic field. An everyday example is a refrigerator magnet used to hold notes on a refrigerator door. Materials that can be magnetized, which are also the ones that are strongly attracted to a magnet, are called ferromagnetic (or ferrimagnetic). These include the elements iron, nickel and cobalt and their alloys, some alloys of rare-earth metals, and some naturally occurring minerals such as lodestone. Although ferromagnetic (and ferrimagnetic) materials are the only ones attracted to a magnet strongly enough to be commonly considered magnetic, all other substances respond weakly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form reaction intermediate, intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous catalysis, homogeneou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |