|
Mannose Receptor
The mannose receptor (Cluster of Differentiation 206, CD206) is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205). The receptor recognises terminal mannose, ''N''-acetylglucosamine and fucose residues on glycans attached to proteins found on the surface of some microorganisms, playing a role in both the innate and adaptive immune systems. Additional functions include clearance of glycoproteins from circulation, including sulphated glycoprotein hormones and glycoproteins released in response to pathological events. The mannose receptor recycles continuously between the plasma membrane and endosomal compartments in a clathrin-dependent manner. Structure Domain organisation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * CD302 (C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pathology
Pathology is the study of the causes and effects of disease or injury. The word ''pathology'' also refers to the study of disease in general, incorporating a wide range of biology research fields and medical practices. However, when used in the context of modern medical treatment, the term is often used in a narrower fashion to refer to processes and tests that fall within the contemporary medical field of "general pathology", an area which includes a number of distinct but inter-related medical specialties that diagnose disease, mostly through analysis of tissue, cell, and body fluid samples. Idiomatically, "a pathology" may also refer to the predicted or actual progression of particular diseases (as in the statement "the many different forms of cancer have diverse pathologies", in which case a more proper choice of word would be " pathophysiologies"), and the affix ''pathy'' is sometimes used to indicate a state of disease in cases of both physical ailment (as in cardiomy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homologous Chromosomes
A couple of homologous chromosomes, or homologs, are a set of one maternal and one paternal chromosome that pair up with each other inside a cell during fertilization. Homologs have the same genes in the same loci where they provide points along each chromosome which enable a pair of chromosomes to align correctly with each other before separating during meiosis. This is the basis for Mendelian inheritance which characterizes inheritance patterns of genetic material from an organism to its offspring parent developmental cell at the given time and area. Overview Chromosomes are linear arrangements of condensed deoxyribonucleic acid (DNA) and histone proteins, which form a complex called chromatin. Homologous chromosomes are made up of chromosome pairs of approximately the same length, centromere position, and staining pattern, for genes with the same corresponding loci. One homologous chromosome is inherited from the organism's mother; the other is inherited from the organism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding (or signal sensing) in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway. When signaling pathways interact with one another they form networks, which allow cellular responses to be coordinated, often by combinatorial signaling events. At the molecular level, such responses include changes in the transcription or translation of genes, and post-translational and conformational changes in proteins, as well as changes in their location. These molecular events are the basic mechanisms controlling cell growth, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibronectin Type II Domain
Fibronectin type II domain is a collagen-binding protein domain. Fibronectin is a multi-domain glycoprotein, found in a soluble form in plasma, and in an insoluble form in loose connective tissue and basement membranes, that binds cell surfaces and various compounds including collagen, fibrin, heparin, DNA, and actin. Fibronectins are involved in a number of important functions e.g., wound healing; cell adhesion; blood coagulation; cell differentiation and migration; maintenance of the cellular cytoskeleton; and tumour metastasis. The major part of the sequence of fibronectin consists of the repetition of three types of domains, which are called type I, II, and III. Type II domain is approximately sixty amino acids long, contains four conserved cysteines involved in disulfide bonds and is part of the collagen-binding region of fibronectin. Type II domains occur two times in fibronectin. Type II domains have also been found in a range of proteins including blood coagulation fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
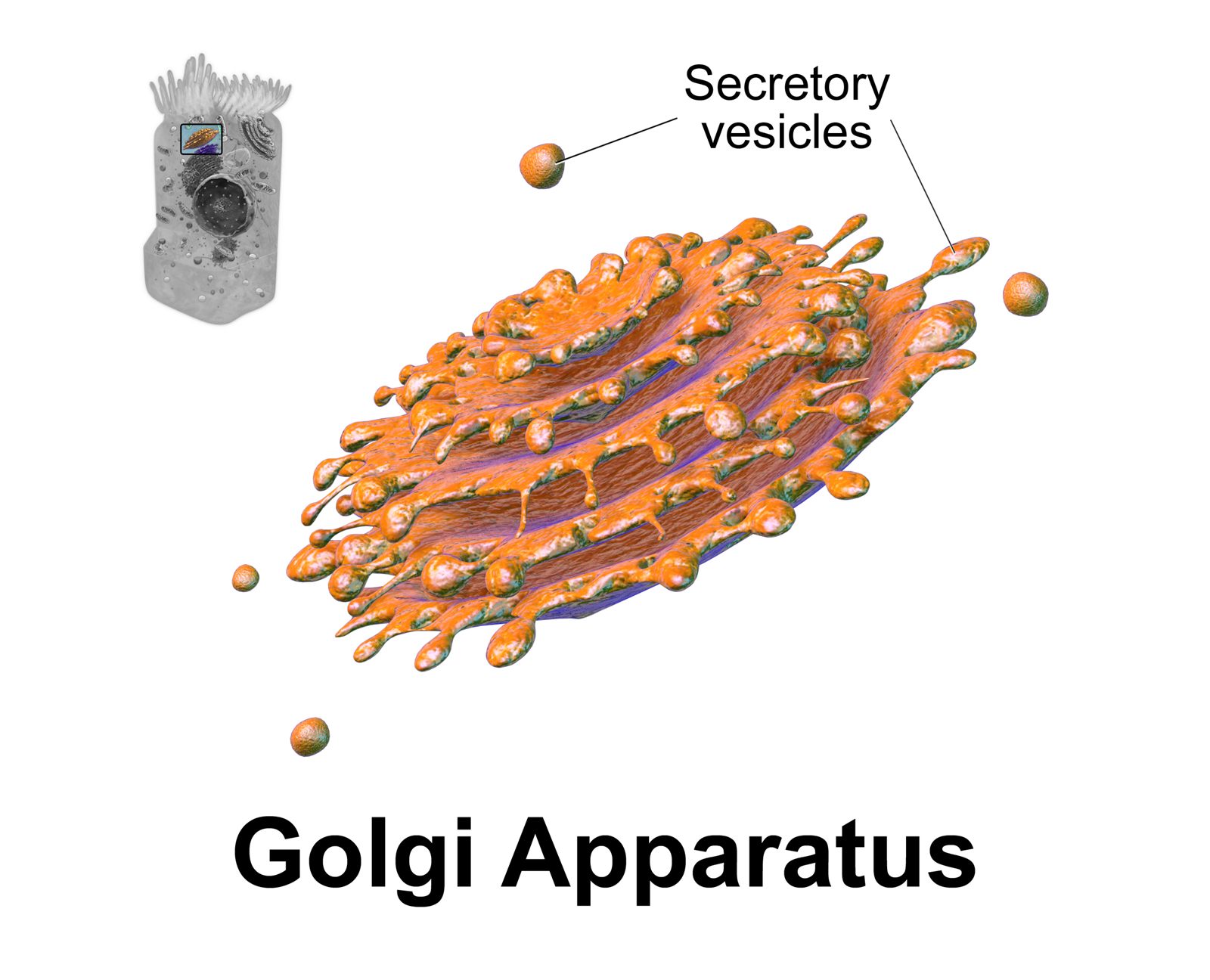
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Protein
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannose Receptor Domain Organisation
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism. Mannose is not an essential nutrient; it can be produced in the human body from glucose, or converted into glucose. Mannose provides 2–5 kcal/g. It is partially excreted in the urine. Etymology The root of both "mannose" and "mannitol" is manna, which the Bible describes as the food supplied to the Israelites during their journey in the region of Sinai. Several trees and shrubs can produce a substance called manna, such as the "manna tree" (''Fraxinus ornus'') from whose secretions mannitol was originally isolated. Structure Mannose commonly exists as two different-sized rings, the pyranose (six-membered) form and the furanose (five-membered) form. Each ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)