|
Malonic Ester Synthesis
The malonic ester synthesis is a chemical reaction where diethyl malonate or another ester of malonic acid is alkylated at the carbon alpha (directly adjacent) to both carbonyl groups, and then converted to a substituted acetic acid. The major drawback of malonic ester synthesis is that the alkylation stage can also produce dialkylated structures. This makes separation of products difficult and yields lower. : Mechanism The carbons alpha to carbonyl groups can be deprotonated by a strong base. The carbanion formed can undergo nucleophilic substitution on the alkyl halide, to give the alkylated compound. On heating, the di-ester undergoes thermal decarboxylation, yielding an acetic acid substituted by the appropriate R group. Thus, the malonic ester can be thought of being equivalent to the −CH2COOH synthon. The esters chosen are usually the same as the base used, i.e. ethyl esters with sodium ethoxide. This is to prevent scrambling by transesterification. Variations Di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can also be accomplished with the help of other enzymes, particularly lipases (one example is the lipase E.C.3.1.1.3). Strong acids catalyse the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic. If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products, this means that esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol. Mechanism In the transesterification mechanism, the carbonyl carbon of the starting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
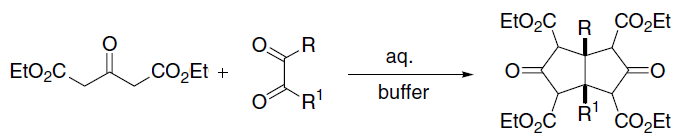
Acetoacetic Ester Synthesis
Acetoacetic ester synthesis is a chemical reaction where ethyl acetoacetate is alkylated at the α-carbon to both carbonyl groups and then converted into a ketone, or more specifically an α-substituted acetone. This is very similar to malonic ester synthesis. : Mechanism A strong base deprotonates the dicarbonyl α-carbon. This carbon is preferred over the methyl carbon because the formed enolate is conjugated and thus resonance stabilized. The carbon then undergoes nucleophilic substitution. When heated with aqueous acid, the newly alkylated ester is hydrolyzed to a β-keto acid, which is decarboxylated to form a methyl ketone. Double deprotonation of ethyl acetoacetate The classical acetoacetatic ester synthesis utilizes the 1:1 conjugate base. Ethyl acetoacetate is however diprotic: :CH3C(O)CH2CO2Et + NaH → CH3C(O)CH(Na)CO2Et + H2 :CH3C(O)CH(Na)CO2Et + BuLi → LiCH2C(O)CH(Na)CO2Et + BuH The dianion (i.e., LiCH2C(O)CH(Na)CO2Et) adds electrophile to the ter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoevenagel Condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence ''condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barbiturate
Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anxiolytic and sedative effects, and are thus controlled in most countries due to the risks associated with such use. Barbiturates have largely been replaced by benzodiazepines and nonbenzodiazepines ("Z-drugs") in routine medical practice, particularly in the treatment of anxiety disorders and insomnia, because of the significantly lower risk of overdose, and the lack of an antidote for barbiturate overdose. Despite this, barbiturates are still in use for various purposes: in general anesthesia, epilepsy, treatment of acute migraines or cluster headaches, acute tension headaches, euthanasia, capital punishment, and assiste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berichte Der Deutschen Chemischen Gesellschaft
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ''Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Henry Perkin, Jr
William is a masculine given name of Norman French origin.Hanks, Hardcastle and Hodges, ''Oxford Dictionary of First Names'', Oxford University Press, 2nd edition, , p. 276. It became very popular in the English language after the Norman conquest of England in 1066,All Things William"Meaning & Origin of the Name"/ref> and remained so throughout the Middle Ages and into the modern era. It is sometimes abbreviated "Wm." Shortened familiar versions in English include Will, Wills, Willy, Willie, Liam, Bill, and Billy. A common Irish form is Liam. Scottish diminutives include Wull, Willie or Wullie (as in Oor Wullie or the play ''Douglas''). Female forms are Willa, Willemina, Wilma and Wilhelmina. Etymology William is related to the German given name ''Wilhelm''. Both ultimately descend from Proto-Germanic ''*Wiljahelmaz'', with a direct cognate also in the Old Norse name ''Vilhjalmr'' and a West Germanic borrowing into Medieval Latin ''Willelmus''. The Proto-Germ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alicyclic Compound
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include bicycloundecane, decalin, and housane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules. Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Cycloalkenes Monocyclic cycloalkenes are cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, cyclooctene, and so on. Bicyclic alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. All Group 1 metals form halides that are white solids at room temperature. A halide ion is a halogen atom bearing a negative charge. The halide anions are fluoride (), chloride (), bromide (), iodide () and astatide (). Such ions are present in all ionic halide salts. Halide minerals contain halides. All these halides are colourless, high melting crystalline solids having high negative enthalpies of form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/PS_2A3_AA_Charges.html, accessed 12/2/2020 The species formed is the conjugate base of that acid. The complementary process, when a proton is added (transferred) to a Brønsted–Lowry base, is protonation (or hydronation). The species formed is the conjugate acid of that base. A species that can either accept or donate a proton is referred to as amphiprotic. An example is the H2O (water) molecule, which can gain a proton to form the hydronium ion, H3O+, or lose a proton, leaving the hydroxide ion, OH−. The relative ability of a molecule to give up a proton is measured by its p''K''a value. A low p''K''a value indicates that the compound is acidic and will easily give up its proton to a bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |