|
Alicyclic Compound
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include bicycloundecane, decalin, and housane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules. Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Cycloalkenes Monocyclic cycloalkenes are cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, cyclooctene, and so on. Bicyclic alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Housane
Housane or bicyclo .1.0entane is a saturated cycloalkane with the formula C5H8. It is a colorless volatile liquid at room temperature. It was named "housane" because of its shape. Structurally, the molecule consists of cyclopropane fused to cyclobutane. The synthesis of molecules containing multiple strained rings, such as housane, is a traditional endeavor in synthetic organic chemistry. Preparation The first synthesis of housane was reported by Criegee in 1957, where housane was obtained from the pyrolysis of 2,3-diazabicyclo .2.1ept-2-ene. : Housane can be prepared in several steps starting with cyclopentadiene. Other methods include photolysis of 2,3-diazabicyclo .2.1ept-2-ene, pyrolysis of ''N''-Phenyl-2-oxo-3-azabicyclo .2.1eptane, and addition of methylene to cyclobutene.P. G. Gassman, K. T. Mansfield "Bicyclo .1.0entane" Org. Synth. 1969, volume 49, pp. 1. Structure and properties The two rings are fused in a ''cis'' configuration—this meso compound formall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared by thermolysis of the ammonium salt 4H7NMe3H. Cyclobutene thermally isomerizes to 1,3-butadiene. This strongly exothermic reaction reflects the dominance of ring strain. In contrast, the corresponding equilibrium for hexafluorocyclobutene disfavors hexafluorobutadiene. See also * Cyclobutane Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercia ... * Cyclobutadiene * Cyclobutyne * Squaric acid References Monomers {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit. Structure and bonding The molecule has a triangular structure. The reduced length of the double bond compared to a single bond causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character: the alkene carbon atoms use sp2.68 hybridization for the ring. Synthesis of cyclopropene and derivatives Early syntheses The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkene
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms and either one or more double bonds, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the ''cis'' isomers, and the term ''cis'' tends to be omitted from the names. Cycloalkenes require considerable p-orbital overlap in the form of a bridge between the carbon-carbon double bond, however, this is not feasible in smaller molecules due to the increase of strain that could break the molecule apart. In greater carbon number cycloalkenes, the addition of CH2 substituents decreases strain. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In larger rings (8 or more atoms), ''cis''–''trans'' isomerism of the double bond may occur. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene Structures
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides. Production and uses Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. In the laboratory, it can be prepared by dehydration of cyclohexanol. : : Reactions and uses Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone. Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam. The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant in the presence of a tungsten catalyst. Bromination gives 1,2-dibromocyclohexane. Structure Cyclohe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leopold Ruzicka
Leopold may refer to: People * Leopold (given name) * Leopold (surname) Arts, entertainment, and media Fictional characters * Leopold (''The Simpsons''), Superintendent Chalmers' assistant on ''The Simpsons'' * Leopold Bloom, the protagonist of James Joyce's ''Ulysses'' * Leopold "Leo" Fitz, a character on the television series ''Agents of S.H.I.E.L.D.'' * Leopold "Butters" Stotch, a character on the television series ''South Park'' * General Leopold von Flockenstuffen, a character in the BBC sitcom Allo 'Allo!'' * Leopold the Cat, Russian cartoon character Other arts, entertainment, and media * Leopold (prize), a biennial German prize for music for children * ''Kate & Leopold'', 2001 romantic comedy film * ''King Leopold's Ghost'', popular history book by Adam Hochschild * " King Leopold's Soliloquy", 1905 pamphlet by Mark Twain. * '' Leopold the Cat'', television series * Léopold Nord & Vous, Belgian musical band Brands and enterprises * Leopold (publisher), a Nethe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
) , image = Nobel Prize.png , alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "MDCCCXXXIII" above, followed by (smaller) "OB•" then "MDCCCXCVI" below. , awarded_for = Outstanding contributions in chemistry , presenter = Royal Swedish Academy of Sciences , location = Stockholm, Sweden , reward = 9 million SEK (2017) , year = 1901 , holder = Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless (2022) , most_awards = Frederick Sanger and Karl Barry Sharpless (2) , website nobelprize.org, previous = 2021 , year2=2022, main= 2022, next= 2023 The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, award ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Wallach
Otto Wallach (; 27 March 1847 – 26 February 1931) was a German chemist and recipient of the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Biography Wallach was born in Königsberg, the son of a Prussian civil servant. His father, Gerhard Wallach, descended from a Jewish family that had converted to Lutheranism. His mother, Otillie (Thoma), was an ethnic German of Protestant religion. Wallach's father was transferred to Stettin (Szczecin) and later to Potsdam. Otto Wallach went to school, a ''Gymnasium'', in Potsdam, where he learned about literature and the history of art, two subjects he was interested his whole life. At this time he also started private chemical experiments at the house of his parents. In 1867 he started studying chemistry at the University of Göttingen, where at this time Friedrich Wöhler was head of organic chemistry. After one semester at the University of Berlin with August Wilhelm von Hofmann, Wallach received his Doctoral degree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baldwin's Rules
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976. Baldwin's rules discuss the relative rates of ring closures of these various types. These terms are not meant to describe the absolute probability that a reaction will or will not take place, rather they are used in a relative sense. A reaction that is disfavoured (slow) does not have a rate that is able to compete effectively with an alternative reaction that is favoured (fast). However, the disfavoured product may be observed, if no alternate reactions are more favoured. The rules classify ring closures in three ways: *the number of atoms in the newly formed ring *into ''exo'' and ''endo'' ring closures, depending whether the bond broken during the ring closure is inside (''endo'') or outside (''exo'') the ring that is being formed *into ''tet'', ''trig'' and ''dig'' geometry of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compound
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like biphenyl that have no connecting atoms, from ''fused ring compounds'' like decalin having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
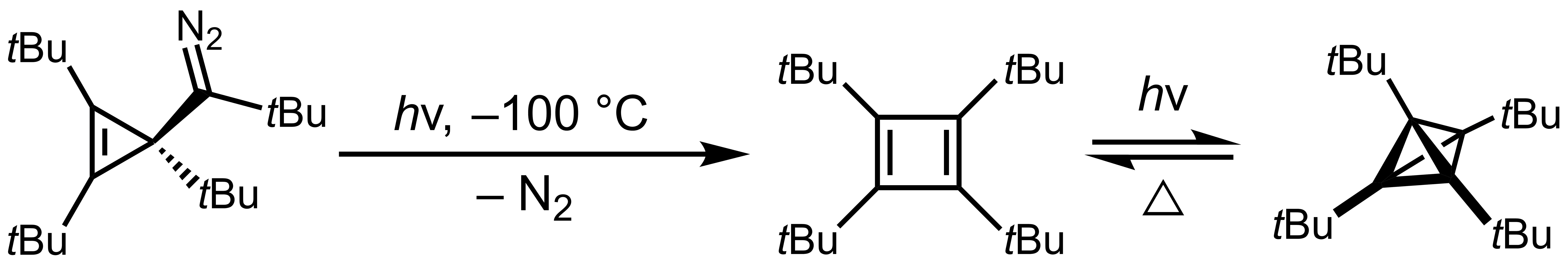
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. Organic tetrahedranes In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |