|
Cycloalkene
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms and either one or more double bonds, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the ''cis'' isomers, and the term ''cis'' tends to be omitted from the names. Cycloalkenes require considerable p-orbital overlap in the form of a bridge between the carbon-carbon double bond, however, this is not feasible in smaller molecules due to the increase of strain that could break the molecule apart. In greater carbon number cycloalkenes, the addition of CH2 substituents decreases strain. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In larger rings (8 or more atoms), ''cis''–''trans'' isomerism of the double bond may occur. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
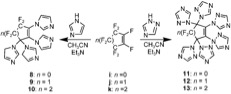
Perfluorocycloalkene
A perfluorocycloalkene (PFCA) fluorocarbon structure with a cycloalkene core. PFCAs have shown reactivity with a wide variety of nucleophiles including phenoxides, alkoxides, organometallic, amines, thiols, and azoles. They or their derivatives are reported to have nonlinear optical activity, and be useful as lubricants, etching agents, components of fuel cells, low dielectric materials, and super hydrophobic and oleophobic coatings. File:Tetrafluorocyclopropene.png, Tetrafluorocyclopropene File:Hexafluorocyclobutene.png, Hexafluorocyclobutene File:Octafluorocyclopentene.png, Octafluorocyclopentene File:Decafluorocyclohexene.png, Decafluorocyclohexene Reactivity Derivatization of these PFCA rings via displacement of fluorine atoms with nucleophiles occurs through an addition-elimination reaction in the presence of a base. Attack of the nucleophile on the PFCA ring generates a carbanion which can eliminate a fluoride ion, resulting in vinyl substituted and allyl substituted prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
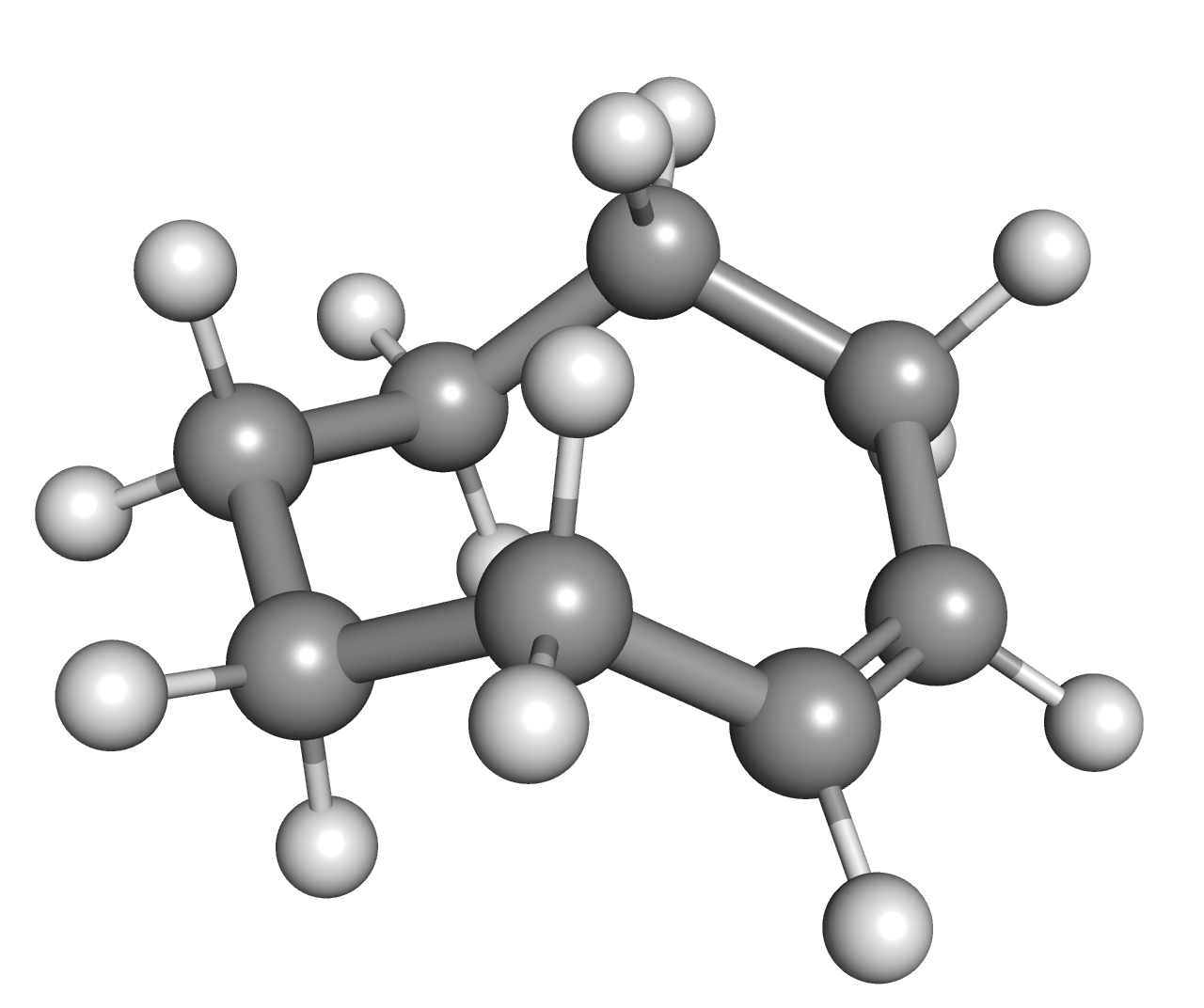
Cis-Cyclooctene
''cis''-Cyclooctene is a cycloalkene with the formula (CH2)6(CH)2. It is a colorless liquid that is used industrially to produce a polymer. It is also a ligand in organometallic chemistry. Cyclooctene is the smallest cycloalkene that can be isolated as both the ''cis''- and ''trans''-isomer. ''cis''-Cyclooctene is shaped like the 8-carbon equivalent chair conformation of cyclohexane. Uses and reactions Cyclooctene undergoes ring-opening metathesis polymerization to give polyoctenamers, which are marketed under the name Vestenamer. ''cis''-Cyclooctene (COE) is a substrate known for quite selectively forming the epoxide, as compared to other cycloalkenes, e.g. cyclohexene. Low amounts of radical by-products are found only. This behaviour is attributed to the difficulty of functionalizing allylic CH centers, which almost orthogonal allylic C-H bonds. Therefore, if radicals are around, they tend to form epoxide via an addition-elimination mechanism. It is used as an easily ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit. Structure and bonding The molecule has a triangular structure. The reduced length of the double bond compared to a single bond causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character: the alkene carbon atoms use sp2.68 hybridization for the ring. Synthesis of cyclopropene and derivatives Early syntheses The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-Cyclooctene
''trans''-Cyclooctene is a cyclic hydrocarbon with the formula ��(CH2)6CH=CH– where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor. Cyclooctene is notable as the smallest cycloalkene that is readily isolated as its ''trans''- isomer. The ''cis''-isomer is much more stable; the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively.Ron Walker, Rosemary M. Conrad, and Robert H. Grubbs (2009): "The living ROMP of ''trans''-cyclooctene". ''Macromolecules'', volume 42, issue 3, pages 599–605. A planar arrangement of the ring carbons would be too strained, and therefore the stable conformations of the ''trans'' form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring. A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides. Production and uses Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. In the laboratory, it can be prepared by dehydration of cyclohexanol. : : Reactions and uses Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone. Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam. The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant in the presence of a tungsten catalyst. Bromination gives 1,2-dibromocyclohexane. Structure Cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared by thermolysis of the ammonium salt 4H7NMe3H. Cyclobutene thermally isomerizes to 1,3-butadiene. This strongly exothermic reaction reflects the dominance of ring strain. In contrast, the corresponding equilibrium for hexafluorocyclobutene disfavors hexafluorobutadiene. See also * Cyclobutane Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercia ... * Cyclobutadiene * Cyclobutyne * Squaric acid References Monomers {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloheptene
Cycloheptene is a 7-membered cycloalkene with a flash point of −6.7 °C. It is a raw material in organic chemistry and a monomer in polymer synthesis. Cycloheptene can exist as either the ''cis''- or the ''trans''-isomer. : ''trans''-Cycloheptene With cycloheptene, the ''cis''-isomer is always assumed but the ''trans''-isomer does also exist. One procedure for the organic synthesis of ''trans''-cycloheptene is by singlet photosensitization of cis-cycloheptene with methyl benzoate and ultraviolet light at −35 °C. The double bond in the ''trans'' isomer is very strained. The directly attached atoms on a simple alkene are all coplanar. In ''trans''-cycloheptene, however, the size of the ring makes it impossible for the alkene and its two attached carbons to have this geometry because the remaining three carbons could not reach far enough to close the ring (see also Bredt's rule). There would have to be unusually large angles (angle strain), unusually long bond-leng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared by thermolysis of the ammonium salt 4H7NMe3H. Cyclobutene thermally isomerizes to 1,3-butadiene. This strongly exothermic reaction reflects the dominance of ring strain. In contrast, the corresponding equilibrium for hexafluorocyclobutene disfavors hexafluorobutadiene. See also * Cyclobutane Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercia ... * Cyclobutadiene * Cyclobutyne * Squaric acid References Monomers {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of the principal cycloalkenes. Production Cyclopentene is produced industrially in large amounts by steam cracking of naphtha. In the laboratory, it is prepared by dehydration of cyclopentanol. It can also produced by the catalytic hydrogenation of cyclopentadiene Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca ....D. Hönicke, R. Födisch, P. Claus, M. Olson: ''Cyclopentadiene and Cyclopentene'', in: '' Ullmanns Enzyklopädie der Technischen Chemie'' 2002, Wiley-VCH, Weinheim. Use in mechanistic organic chemistry Cyclopentene is used in an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |