|
Kedarcidin
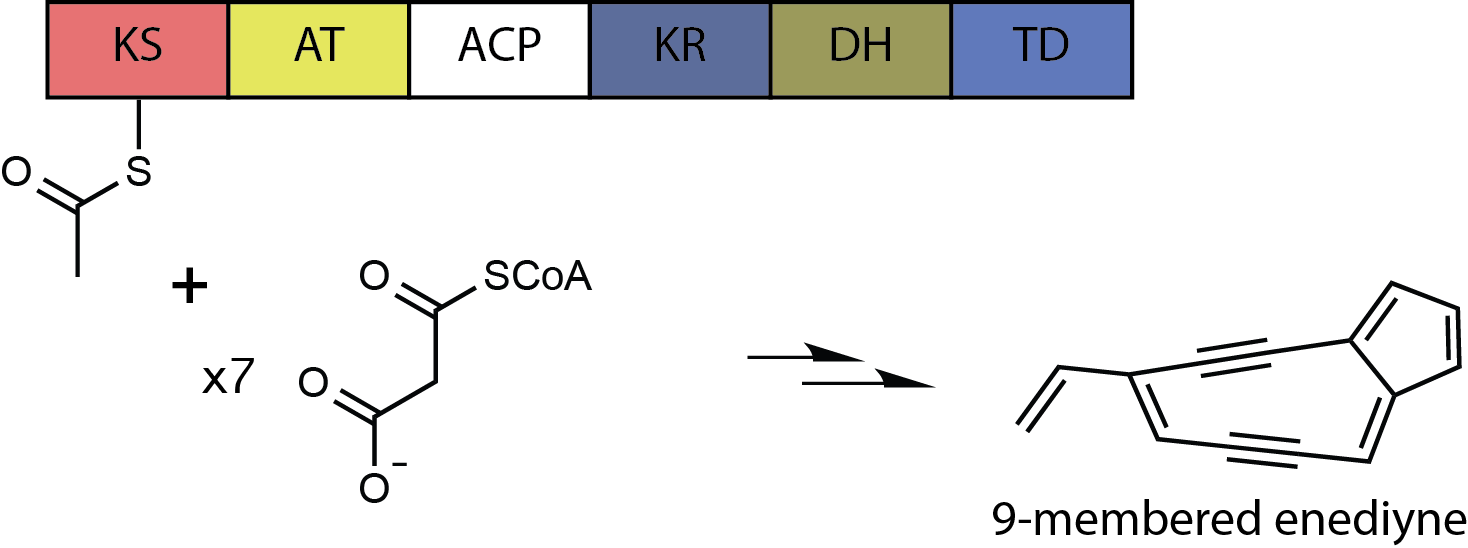
Kedarcidin is a chromoprotein antitumor antibiotic first isolated from an Actinomycete in 1992, comprising an ansa-bridged enediyne chromophore (shown) as well as an apoprotein that serves to stabilize the toxin in the Actinomycete. Like other members of the enediyne class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene directly attached to two alkynyl appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism. Because it achieves this by causing DNA damage, however, kedarcidin is capable of harming tumor cells, as well. Kedarcidin is thus the subject of scientific research, both for its structural complexity as well as its anticancer properties. Discovery and structure elucidation Kedarcidin was first discovered in 1992 when bioassays conducted at Bristol-Myers Squibb indicated the presence of a DNA-damaging chromoprotein in the fermentation broth of an Actinomycete strain. The involvement of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kedarcidin Structure Series Of Revisions
Kedarcidin is a chromoprotein antitumor antibiotic first isolated from an Actinomycete in 1992, comprising an ansa-bridged enediyne chromophore (shown) as well as an apoprotein that serves to stabilize the toxin in the Actinomycete. Like other members of the enediyne class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene directly attached to two alkynyl appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism. Because it achieves this by causing DNA damage, however, kedarcidin is capable of harming tumor cells, as well. Kedarcidin is thus the subject of scientific research, both for its structural complexity as well as its anticancer properties. Discovery and structure elucidation Kedarcidin was first discovered in 1992 when bioassays conducted at Bristol-Myers Squibb indicated the presence of a DNA-damaging chromoprotein in the fermentation broth of an Actinomycete strain. The involvement of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kedarcidin Core Bergman Equilibrium
Kedarcidin is a chromoprotein antitumor antibiotic first isolated from an Actinomycete in 1992, comprising an ansa-bridged enediyne chromophore (shown) as well as an apoprotein that serves to stabilize the toxin in the Actinomycete. Like other members of the enediyne class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene directly attached to two alkynyl appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism. Because it achieves this by causing DNA damage, however, kedarcidin is capable of harming tumor cells, as well. Kedarcidin is thus the subject of scientific research, both for its structural complexity as well as its anticancer properties. Discovery and structure elucidation Kedarcidin was first discovered in 1992 when bioassays conducted at Bristol-Myers Squibb indicated the presence of a DNA-damaging chromoprotein in the fermentation broth of an Actinomycete strain. The involvement of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromoprotein
A chromoprotein is a conjugated protein that contains a pigmented prosthetic group (or cofactor). A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other examples of chromoproteins include other hemochromes, cytochromes, phytochromes and flavoproteins. In hemoglobin there exists a chromoprotein (tetramer MW:4 x 16.125 =64.500), namely heme, consisting of Fe++ four pyrrol rings. A single chromoprotein can act as both a phytochrome and a phototropin due to the presence and processing of multiple chromophores. Phytochrome in ferns contains PHY3 which contains an unusual photoreceptor with a dual-channel possessing both phytochrome (red-light sensing) and phototropin (blue-light sensing) and this helps the growth of fern plants at low sunlight. The GFP protein family includes both fluorescent proteins and non-fluorescent chromoproteins. Through mutagenesis or irradiation, the non-fluorescent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intercalation (chemistry)
In chemistry, intercalation is the reversible inclusion or insertion of a molecule (or ion) into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides. : Examples Graphite One famous intercalation host is graphite, which intercalates potassium as a guest. Intercalation expands the van der Waals gap between sheets, which requires energy. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e., redox. Two potassium graphite compounds are KC8 and KC24. Carbon fluorides (e.g., (CF)x and (C4F)) are prepared by reaction of fluorine with graphitic carbon. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent, thus fluorine is not intercalated. Such materials have been considered as a cathode in various lithium batteries. Treating graphite with strong acids in the presence of oxidizing agents causes the graphite to oxidise. Graphite bisulfate, 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium ''Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin (marketed as Besponsa) an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neocarzinostatin
Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by ''Streptomyces macromomyceticus''. It consists of two parts, a labile chromophore (the non-protein molecular entity shown at right) and a 113 amino acid protein to which the chromophore is tightly and non- covalently bound with high affinity (Kd ~ 10−10 M). The non-protein component is a very potent DNA-damaging agent; However it is extremely unstable and the role of the protein is to protect it and release it to the target DNA. Opening of the epoxide under reductive conditions present in cells creates favorable conditions for a Masamune-Bergman cyclization, leading to formation of benzyne, followed by DNA strand cleavage. Another important member of the chromoprotein group of natural products is kedarcidin. As a medicine it is among the most potent, and in Japan only it has been used against liver cancer clinically. __TOC__ Biosynthesis of NCS Chromophore The biosynthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. Discovered in 1929 by Phoebus Levene, deoxyribose is most notable for its presence in DNA. Since the pentose sugars arabinose and ribose only differ by the stereochemistry at C2′, 2-deoxyribose and 2-deoxyarabinose are equivalent, although the latter term is rarely used because ribose, not arabinose, is the precursor to deoxyribose. Structure Several isomers exist with the formula H−(C=O)−(CH2)−(CHOH)3−H, but in deoxyribose all the hydroxyl groups are on the same side in the Fischer projection. The term "2-deoxyribose" may refer to either of two enantiomers: the biologically important -2-deoxyribose and to the rarely encountered mirror image -2-deoxyribose.C Bernelot-Moens and B Demple (1989), ''Multiple DNA repair activities f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bergman Cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor (''Scheme 1''). It is the most famous and well-studied member of the general class of cycloaromatization reactions. It is named for Japanese-American chemist Satoru Masamune (b. 1928) and American chemist Robert G. Bergman (b. 1942). The reaction product is a derivative of benzene. The reaction proceeds by a thermal reaction or pyrolysis (above 200 °C) forming a short-lived and very reactive para-benzyne biradical species. It will react with any hydrogen donor such as 1,4-cyclohexadiene which converts to benzene. When quenched by tetrachloromethane the reaction product is a 1,4-dichlorobenzene and with methanol the reaction product is benzyl alcohol. When the enyne moiety is incorporated into a 10-membered hydrocarbon rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Amino Acid
Beta-peptides (β-peptides) are peptides derived from β-amino acids, in which the amino group is attached to the β-carbon (i.e. the carbon two atoms away from the carboxylate group). The parent β-amino acid is β-alanine (H2NCH2CH2CO2H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds. β-peptides usually do not occur in nature. β-peptide-based antibiotics are being explored as ways of evading antibiotic resistance. Early studies in this field were published in 1996 by the group of Dieter Seebach and that of Samuel Gellman. Structure As there are two carbons available for substitution, β-amino acids have four sites (chirality included; as opposed to two in α-amino acids) for attaching the organic residue group. Accordingly, two main types β-amino acids exist differing by which carbon the residue is attached to: ones with the organic residue (R) next to the amine are called β3 and those with position next to the carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |