|
Iron Homeostasis
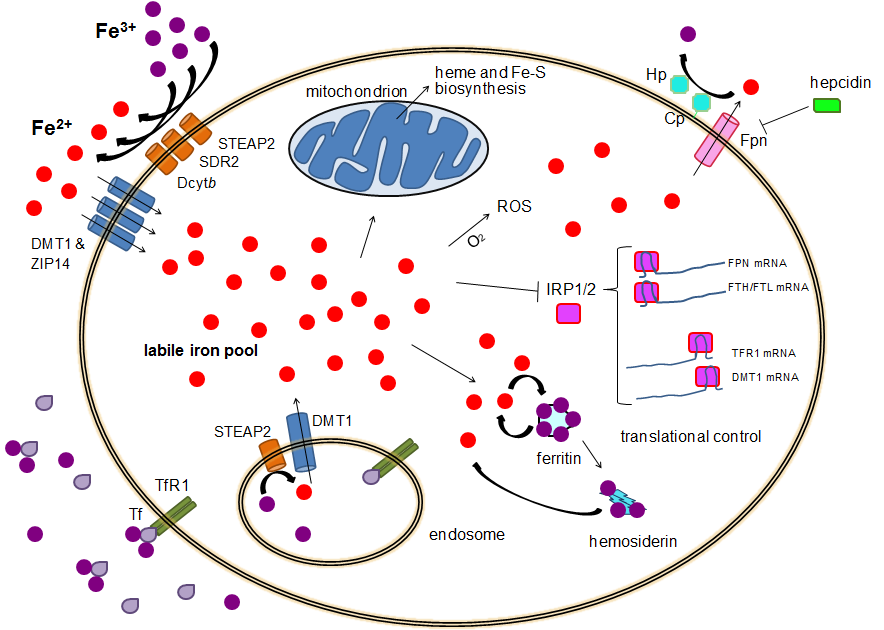
Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia. Importance of iron regulation Iron is an essential bioelement for most forms of life, from bacteria to mammals. Its importance lies in its ability to mediate electron transfer. In the ferrous state (Fe2+), iron acts as an electron donor, while in the ferric state (Fe3+) it acts as an acceptor. Thus, iron plays a vital role in the cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellular Iron Homeostasis
Cellular may refer to: *Cellular automaton, a model in discrete mathematics *Cell biology, the evaluation of cells work and more *Cellular (film), ''Cellular'' (film), a 2004 movie *Cellular frequencies, assigned to networks operating in cellular RF bands *Cellular manufacturing *Cellular network, cellular radio networks *U.S. Cellular Field, also known as "The Cell", a baseball stadium in Chicago *U.S. Cellular Arena, an arena in Milwaukee, Wisconsin Terms such as cellular organization, cellular structure, cellular system, and so on may refer to: *Cell biology, the evaluation of how cells work and more *Cellular communication networks, systems for allowing communication through mobile phones and other mobile devices *Cellular organizational structures, methods of human organization in social groups *Clandestine cell organizations, entities organized to commit crimes, acts of terror, or other malicious activities See also *Cell (other) {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
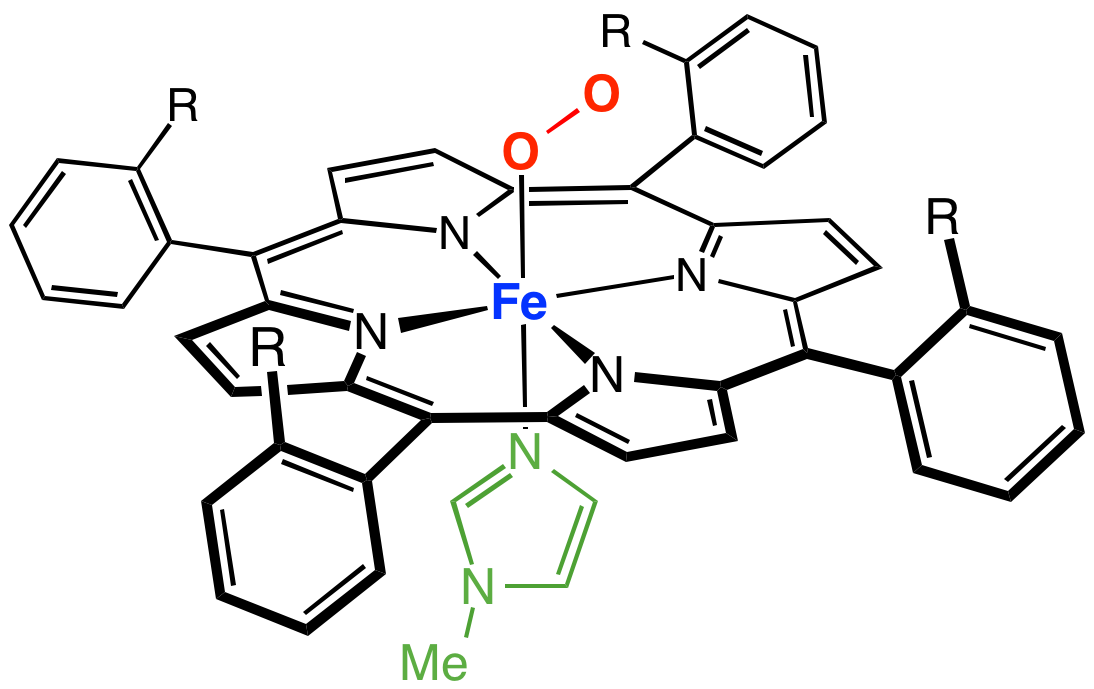
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobin has a higher affinity for oxygen and does not have cooperative binding with oxygen like hemoglobin does. In humans, myoglobin is only found in the bloodstream after muscle injury. (Google books link is the 2008 edition) High concentrations of myoglobin in muscle cells allow organisms to hold their breath for a longer period of time. Diving mammals such as whales and seals have muscles with particularly high abundance of myoglobin. Myoglobin is found in Type I muscle, Type II A, and Type II B; although many texts consider myoglobin not to be found in smooth muscle, this has proved erroneous: there is also myoglobin in smooth muscle cells. Myoglobin was the first protein to have its three-dimensional structure revealed by X-ray crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lung
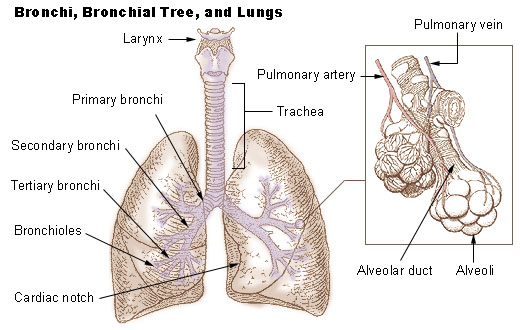
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the air and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their different muscles to support and foster breathing. In earlier tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the main muscle of respiration that drives breathing is the diaphragm. The lungs also provide airflow that makes vocal sounds including human speech possible. Humans have two lungs, one on the left and on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemiosmosis
Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis. Hydrogen ions, or protons, will diffuse from a region of high proton concentration to a region of lower proton concentration, and an electrochemical concentration gradient of protons across a membrane can be harnessed to make ATP. This process is related to osmosis, the movement of water across a selective membrane, which is why it is called "chemiosmosis". ATP synthase is the enzyme that makes ATP by chemiosmosis. It allows protons to pass through the membrane and uses the free energy difference to phosphorylate adenosine diphosphate (ADP), making ATP. The generation of ATP by chemiosmosis occurs in mitochondria and chloroplasts, as well as in most bacteria and archaea. For instan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP Synthase
ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation of P-O bond (phosphodiester bond). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is: * ADP + Pi + 2H+out ATP + H2O + 2H+in The formation of ATP from ADP and Pi is energetically unfavorable and would normally proceed in the reverse direction. In order to drive this reaction forward, ATP synthase couples ATP synthesis during cellular respiration to an electrochemical gradient created by the difference in proton (H+) concentration across the inner mitochondrial membrane in eukaryotes or the plasma membrane in bacteria. During photosynthesis in plants, ATP is synthesized by ATP synthase using a proton gradient created in the thylakoid lumen through the thylakoid membrane and into the chloroplast stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and the electrical gradient, or difference in charge across a membrane. When there are unequal concentrations of an ion across a permeable membrane, the ion will move across the membrane from the area of higher concentration to the area of lower concentration through simple diffusion. Ions also carry an electric charge that forms an electric potential across a membrane. If there is an unequal distribution of charges across the membrane, then the difference in electric potential generates a force that drives ion diffusion until the charges are balanced on both sides of the membrane. Electrochemical gradients are essential to the operation of batteries and other electrochemical cells, photosynthesis and cellular respiration, and certain other bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transport Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that transferred from NADH and FADH2 to the ETC involves 4 multi-subunit large enzymes complexes and 2 mobile electron carriers. Many of the enzymes in the electron transport chain are membrane-bound. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cristae
A crista (; plural cristae) is a fold in the inner membrane of a mitochondrion. The name is from the Latin for ''crest'' or ''plume'', and it gives the inner membrane its characteristic wrinkled shape, providing a large amount of surface area for chemical reactions to occur on. This aids aerobic cellular respiration, because the mitochondrion requires oxygen. Cristae are studded with proteins, including ATP synthase and a variety of cytochromes. Background With the discovery of the dual-membrane nature of mitochondria, the pioneers of mitochondrial ultrastructural research proposed different models for the organization of the mitochondrial inner membrane. Three models proposed were: *Baffle model – According to Palade (1953), the mitochondrial inner membrane is convoluted in a baffle-like manner with broad openings towards the intra-cristal space. This model entered most textbooks and was widely believed for a long time. *Septa model – Sjöstrand (1953) suggested that s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis. The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle producing carbon dioxide, and the energetic electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a series of electron acceptors in a series of redox reactions ending in oxygen, who ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |