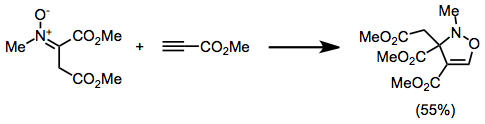|
Isoxazoline
Isoxazolines are a class of five-membered heterocyclic chemical compounds, containing one atom each of oxygen and nitrogen which are located adjacent to one another. The ring was named in-line with the Hantzsch–Widman nomenclature. They are structural isomers of the more common oxazolines and exist in three different isomers depending on the location of the double bond. The relatively weak N-O bond makes isoxazolines prone to ring-opening and rearrangement reactions. Compounds containing an isoxazoline ring, sometimes referred to isoxazolyls, have a variety of uses with many being biologically active. A number of naturally occurring isoxazolines with possible anti-cancer activity are produced by marine sponges. Perhaps the most commonly encountered products containing isoxazolines are some veterinary medicines used to prevent flea infestations in dogs e.g. Fluralaner, Afoxolaner and Sarolaner. Synthesis 2-Isoxazolines are generally produced by the 1,3-dipolar cycloaddition of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Afoxolaner
Afoxolaner (INN) is an insecticide and acaricide that belongs to the isoxazoline chemical compound group. It acts as an antagonist at ligand-gated chloride channels, in particular those gated by the neurotransmitter gamma-aminobutyric acid ( GABA-receptors). Isoxazolines, among the chloride channel modulators, bind to a distinct and unique target site within the insect GABA-gated chloride channels, thereby blocking pre-and post- synaptic transfer of chloride ions across cell membranes. Prolonged afoxolaner-induced hyperexcitation results in uncontrolled activity of the central nervous system and death of insects and acarines. Marketing Afoxolaner is the active principle of the veterinary medicinal products NexGard (alone), Frontpro (alone) and Nexgard Spectra (in combination with milbemycin oxime). They are indicated for the treatment and prevention of flea infestations, and the treatment and control of tick infestations in dogs and puppies (8 weeks of age and older, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluralaner
Fluralaner (INN) is a systemic insecticide and acaricide that is administered orally or topically. The U.S. Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014 and Bravecto Plus as a topical treatment for cats in November 2019, with warnings about possible side effects in both species. The EU approved the drug in February 2014. Australia approved it for the treatment and prevention of ticks and fleas on dogs in January 2015. Mode of action Fluralaner inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABA receptors) and L-glutamate-gated chloride channels (GluCls). Potency of fluralaner is comparable to fipronil Fipronil is a broad-spectrum insecticide that belongs to the phenylpyrazole chemical family. Fipronil disrupts the insect central nervous system by blocking the ligand-gated ion channel of the GABAA receptor and glutamate-gated chloride (GluCl ... (a related GABA-antagonist insecticide and ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usuall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarolaner
Sarolaner, sold under the brand name Simparica, is an ectoparasiticide veterinary medication for the treatment of flea and tick infestations in dogs. It is also used off-label to control sarcoptic mange and demodectic mange. Sarolaner is also a component of the combination drug Simparica Trio, which contains sarolaner, moxidectin, and pyrantel. It is used for prevention of heartworm disease caused by ''Dirofilaria immitis''; treat and prevent flea infestations; treat and control tick infestations with the lone star tick, Gulf Coast tick, American dog tick, black-legged tick, and brown dog tick; and treat and control roundworm and adult hookworm infections. Sarolaner is also an ingredient in feline combination antiparasitic Revolution Plus (or Stronghold Plus), which contains sarolaner and selamectin and is used for prevention of sarcoptic mange, feline hookworms, feline roundworms, ear mites, and heartworms, as well as treating and preventing fleas and ticks Ticks ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone-olefin (3+2) Cycloaddition
The nitrone-olefin (3+2) cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a +2cycloaddition process. This reaction is a 1,3-dipolar cycloaddition, in which the nitrone acts as the 1,3-dipole, and the alkene or alkyne as the dipolarophile. Mechanism and stereochemistry When nitrones are combined with either alkenes or alkynes, +2cycloaddition leads to the formation of a new C–C bond and a new C–O bond. The cycloadditions is stereospecific with respect to the configuration of the alkene; however, diastereoselectivity in reactions of C-substituted nitrones is often low. Regioselectivity is controlled by the dominant frontier orbitals interacting during the reaction, and substrates with electronically distinct substituents tend to react with high regioselectivity. Intramolecular versions of the reaction have been used to synthesize complex polyclic carbon frameworks. Reduction of the N–O linkage l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Use Of Directed Cycloaddition In Epothilones Synthesis
{{disambig ...
Use may refer to: * Use (law), an obligation on a person to whom property has been conveyed * Use (liturgy), a special form of Roman Catholic ritual adopted for use in a particular diocese * Use–mention distinction, the distinction between using a word and mentioning it * Consumption (economics) ** Resource depletion, use to the point of lack of supply ** Psychological manipulation, in a form that treats a person is as a means to an end * Rental utilization, quantification of the use of assets to be continuously let See also * Use case * User story * USE (other) * Used (other) * User (other) Ancient Egyptian roles * User (ancient Egyptian official), an ancient Egyptian nomarch (governor) of the Eighth Dynasty * Useramen, an ancient Egyptian vizier also called "User" Other uses * User (computing), a person (or software) using an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazole
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole. Occurrence Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol. Synthesis Isoxazole can be synthesised via a variety of methods. Examples include via a 1,3-dipolar cycloaddition of nitrile oxides with alkynes; or the reaction of hydroxylamine with 1,3-diketones or derivatives of propiolic acid. Photochemistry The photolysis of isoxazole was first reported in 1966. Due to the weak N-O bond, the isoxazole ring tends to collapse under UV irradiation, rearranging to oxazole through azirine intermediate. Meanwhile, the azirine intermediate can react with nucleophiles, especially carboxylic acids. Given the photoreactions, isoxazole group is developed as a native photo-cross-linker for photoaffinity labeling and chemoproteomic studies. Pharmaceuticals and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazolidine
An oxazolidine is a five-membered ring compound consisting of three carbon atoms, a nitrogen atom and an oxygen atom. The O atom and NH group are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon atom between the O and N atoms (or it would be an ''isoxazolidine''). All of the carbon atoms in oxazolidines are reduced (compare to oxazole and oxazoline). Some of their derivatives, the 2,4-Oxazolidinedione, oxazolidinediones, are used as anticonvulsants. Oxazolidines were first synthesized over 100 years ago. Monooxazolidines Oxazolidines that are the precursor to bisoxazolidines are in effect mono-oxazolidines. They are also used as moisture scavengers in polyurethane and other systems. Dioxooxazolidines Oxazolidines where the carbon centers at the 1 and 3 positions are carbonyl group, carbonyls are called dioxooxazolidines. Some of these are commercial fungicides including chlozolinate, vinclozolin, and famoxadone. Bisoxazolidines Bisoxazol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone
In organic chemistry, a nitrone is a functional group consisting of an ''N''-oxide of an imine. The general structure is , where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloadditions to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings. Generation of nitrones Nitrones are generated most often either by the oxidation of hydroxylamines or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide. Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |