|
Guanidinium Chloride
Guanidinium chloride or guanidine hydrochloride, usually abbreviated GdmCl and sometimes GdnHCl or GuHCl, is the hydrochloride salt of guanidine. Structure Guanidinium chloride crystallizes in orthorhombic space group ''Pbca''. The crystal structure consists of a network of guanidinium cations and chloride anions linked by N–H···Cl hydrogen bonds. Acidity Guanidinium chloride is a weak acid with a pKa of 13.6. The reason that it is such a weak acid is the complete delocalisation of the positive charge through 3 nitrogen atoms (plus a little bit positive charge on carbon). However, some stronger bases can deprotonate it, such as sodium hydroxide: C(NH2)3+ + OH- > HNC(NH2)2 + H2O The equilibrium is not complete because the acidity difference between guanidinium and water is not large (The approximate pKa values: 13.6 vs 15.7). Complete deprotonation should be done with extremely strong bases, such as lithium diisopropylamide. C(NH2)3+Cl- + Li+N(C3H7)2- -> HNC(NH2)2 + H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Orthorhombic Crystal System
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Protein Folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Guanidinium Compounds
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experiencing renal failure. A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine. Structure Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a group. Isobutene can be seen as the carbon analogue in much the same way. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule. In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction. Production Guanidine can be obtained from natural sources, being first isol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Lambert–Eaton Myasthenic Syndrome
Lambert–Eaton myasthenic syndrome (LEMS) is a rare autoimmune disorder characterized by muscle weakness of the limbs. Around 60% of those with LEMS have an underlying malignancy, most commonly small-cell lung cancer; it is therefore regarded as a paraneoplastic syndrome (a condition that arises as a result of cancer elsewhere in the body). It is the result of antibodies against presynaptic voltage-gated calcium channels, and likely other nerve terminal proteins, in the neuromuscular junction (the connection between nerves and the muscle that they supply). The diagnosis is usually confirmed with electromyography and blood tests; these also distinguish it from myasthenia gravis, a related autoimmune neuromuscular disease. If the disease is associated with cancer, direct treatment of the cancer often relieves the symptoms of LEMS. Other treatments often used are steroids, azathioprine, which suppress the immune system, intravenous immunoglobulin, which outcompetes autoreactive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Ovalbumin
Ovalbumin (abbreviated OVA) is the main protein found in egg white, making up approximately 55% of the total protein. Ovalbumin displays sequence and three-dimensional homology to the serpin superfamily, but unlike most serpins it is not a serine protease inhibitor. The function of ovalbumin is unknown, although it is presumed to be a storage protein. Research Ovalbumin is an important protein in several different areas of research, including: * general studies of protein structure and properties (because it is available in large quantities). * studies of serpin structure and function (the fact that ovalbumin does not inhibit proteases means that by comparing its structure with that of inhibitory serpins, the structural characteristics required for inhibition can be determined). * proteomics (chicken egg ovalbumin is commonly used as a molecular weight marker for calibrating electrophoresis gels). * immunology (commonly used to stimulate an allergic reaction in test subjects; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Sulfhydryl
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. All Group 1 metals form halides that are white solids at room temperature. A halide ion is a halogen atom bearing a negative charge. The halide anions are fluoride (), chloride (), bromide (), iodide () and astatide (). Such ions are present in all ionic halide salts. Halide minerals contain halides. All these halides are colourless, high melting crystalline solids having high negative enthalpies of form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Gelatin
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics. Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Hsp104
Hsp104 is a heat-shock protein Heat shock proteins (HSP) are a family of proteins produced by cell (biology), cells in response to exposure to Stress (biology), stressful conditions. They were first described in relation to heat shock#Heat shock, heat shock, but are now known to .... It is known to reverse toxicity of mutant α-synuclein, TDP-43, FUS, and TAF15 in yeast cells. References Heat shock proteins {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
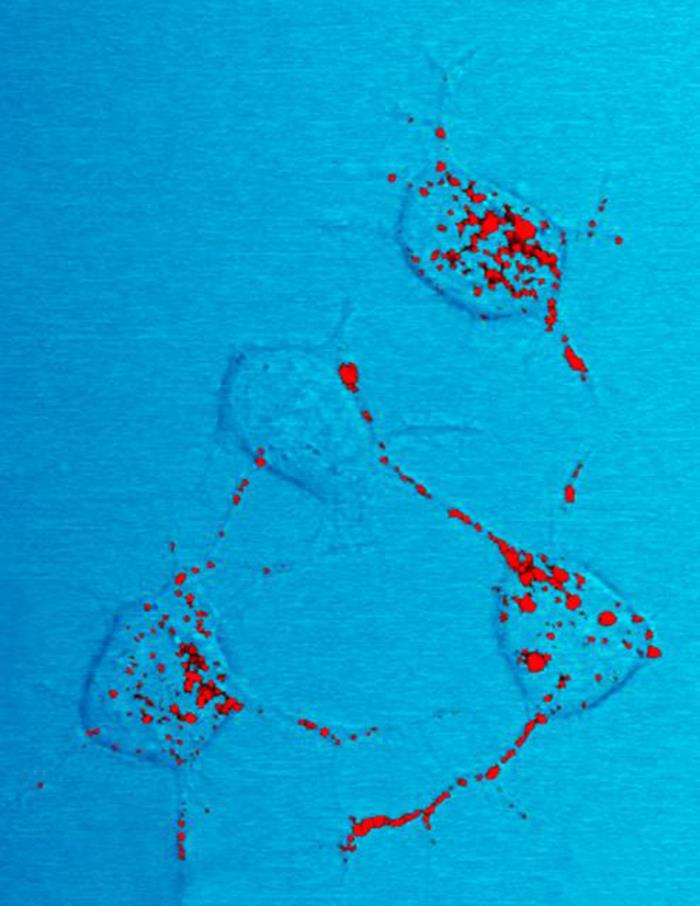 |
Prion
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It is not known what causes a normal protein to misfold, but the resulting abnormal three-dimensional structure confers infectious properties by collapsing nearby protein molecules into the same shape. The word ''prion'' is derived from the term, "proteinaceous infectious particle". In comparison to all other known infectious agents such as viroids, viruses, bacteria, fungi, and parasites, all of which contain nucleic acids ( DNA, RNA, or both), the hypothesized role of a protein as an infectious agent stands in contrast. Prion isoforms of the prion protein (PrP), whose specific function is uncertain, are hypothesized as the cause of transmissible spongiform encephalopathies (TSEs), including scrapie in sheep, chronic wasting disease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Random Coil
In polymer chemistry, a random coil is a conformation of polymers where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the chains in a population of macromolecules. The conformation's name is derived from the idea that, in the absence of specific, stabilizing interactions, a polymer backbone will "sample" all possible conformations randomly. Many unbranched, linear homopolymers — in solution, or above their melting temperatures — assume (approximate) random coils. Random walk model: The Gaussian chain There are an enormous number of different ways in which a chain can be curled around in a relatively compact shape, like an unraveling ball of twine with much open space, and comparatively few ways it can be more or less stretched out. So, if each conformation has an equal probability or statistical weight, chains are much more likely to be ball-like th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Protein Structure
Protein structure is the molecular geometry, three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per chemical reaction, reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |