|
Ethyl Benzene
Ethylbenzene is an organic compound with the formula . It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as an reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene. Occurrence and applications Ethylbenzene occurs naturally in coal tar and petroleum. The dominant application of ethylbenzene is its role as an intermediate in the production of polystyrene. Catalytic dehydrogenation of ethylbenzene gives hydrogen and styrene: : → C6H5CH=CH2 + As of May 2012, more than 99% of all the ethylbenzene produced is used for this purpose. Ethylbenzene hydroperoxide, a reagent and radical initiator, is produced by autoxidation of ethylbenzene: :C6H5CH2CH3 + O2 → C6H5CH(O2H)CH3 Niche uses Ethylbenzene is added to gasoline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is given as charge times length of separation, it is a vector whose direction is in the direction of the unit vector of the position vector of the positive charge w.r.t negative charge: :p = ''q''r. named in honour of the physicist Peter J. W. Debye. It is defined as statcoulomb-centimeters.The statcoulomb is also known as the franklin or electrostatic unit of charge. :1 statC = 1 Fr = 1 esu = 1 cm3/2⋅g1/2⋅s−1. Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10−10 statcoulomb10−10 statcoulomb corresponds to approximately 0.2083 units of elementary charge. (generally called e.s.u. (electrostatic unit) in older scientific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organic compounds in air at ambient temperatures. Many common phenomena can be attributed to autoxidation, such as food going rancid, the 'drying' of varnishes and paints, and the perishing of rubber. It is also an important concept in both industrial chemistry and biology. Autoxidation is therefore a fairly broad term and can encompass examples of photooxygenation and catalytic oxidation. The common mechanism is a free radical chain reaction, where the addition of oxygen gives rise to hydroperoxides and their associated peroxy radicals (ROO•). Typically, an induction period is seen at the start where there is little activity; this is followed by a gradually accelerating take-up of oxygen, giving an autocatalytic reaction which can only be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
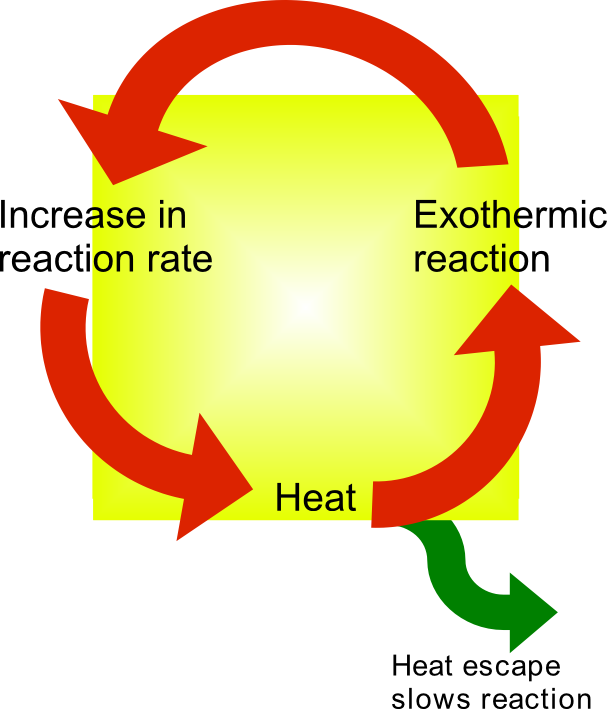
Thermal Runaway
Thermal runaway describes a process that is accelerated by increased temperature, in turn releasing energy that further increases temperature. Thermal runaway occurs in situations where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result. It is a kind of uncontrolled positive feedback. In chemistry (and chemical engineering), thermal runaway is associated with strongly exothermic reactions that are accelerated by temperature rise. In electrical engineering, thermal runaway is typically associated with increased current flow and power dissipation. Thermal runaway can occur in civil engineering, notably when the heat released by large amounts of curing concrete is not controlled. In astrophysics, runaway nuclear fusion reactions in stars can lead to nova and several types of supernova explosions, and also occur as a less dramatic event in the normal evolution of solar-mass stars, the " he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change Δ''G''⚬ is negative." A strongly exothermic reaction will usually also be exergonic because Δ''H''⚬ makes a major contribution to Δ''G''⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Examples Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction: :4Fe + 3O2 → 2Fe2O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shell Plc
Shell plc is a British multinational oil and gas company headquartered in London, England. Shell is a public limited company with a primary listing on the London Stock Exchange (LSE) and secondary listings on Euronext Amsterdam and the New York Stock Exchange. It is one of the oil and gas "supermajors" and by revenue and profits is consistently one of the largest companies in the world. Measured by both its own emissions, and the emissions of all the fossil fuels it sells, Shell was the ninth-largest corporate producer of greenhouse gas emissions in the period 1988–2015. Shell was formed in 1907 through the merger of Royal Dutch Petroleum Company of the Netherlands and The "Shell" Transport and Trading Company of the United Kingdom. The combined company rapidly became the leading competitor of the American Standard Oil and by 1920 Shell was the largest producer of oil in the world. Shell first entered the chemicals industry in 1929. Shell was one of the " Seven Sisters" whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moerdijk
Moerdijk () is a municipality and a town in the South of the Netherlands, in the province of North Brabant. History The municipality of Moerdijk was founded in 1997 following the merger of the municipalities of Fijnaart en Heijningen, Klundert, Standdaarbuiten, Willemstad, and Zevenbergen. At that time the new municipality was called Zevenbergen. The name changed to Moerdijk on 1 April 1998. * List of mayors of Moerdijk Population centres Topography ''Dutch Topographic map of the municipality of Moerdijk, June 2015'' The village of Moerdijk The village of Moerdijk is one of the smaller villages of the municipality. Population as of 2002 is 1,205. ''Moerdijk'' is however a well-known name in the Netherlands, because of the large Moerdijk industrial area, with a large power plant, and because of the well-known Moerdijk bridges (highway and railway bridges) across the Hollands Diep. This was the last bridge available for the retreat from the vital Scheldt Estuary of the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These positive ions can be exchanged for others in a contacting electrolyte solution. exchanged zeolites are particularly useful as solid acid catalysts. The term ''zeolite'' was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is ob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or Cracking (chemistry), cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In Chemical industry, industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylenes
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value. The mixture is referred to as both xylene and, more precisely, xylenes. Mixed xylenes refers to a mixture of the xylenes plus ethylbenzene. The four compounds have identical empirical formulas . Typically the four compounds are produced together by various catalytic reforming and pyrolysis methods. Occurrence and production Xylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. They also occur in crude oil in concentrations of about 0.5–1%, depending on the source. Small quantities occur in gasoline and aircraft f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: rotati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |