|
Emergency-use Authorization
An Emergency Use Authorization (EUA) in the United States is an authorization granted to the Food and Drug Administration (FDA) under sections of the Federal Food, Drug, and Cosmetic Act as added to and amended by various Acts of Congress, including by the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), as codified by , to allow the use of a drug prior to approval. It does not constitute ''approval'' of the drug in the full statutory meaning of the term, but instead authorizes the FDA to facilitate availability of an unapproved product, or an unapproved use of an approved product, during a declared state of emergency from one of several agencies or of a "material threat" by the Secretary of Homeland Security. Use EUAs have historically been infrequent. A review article by Rizk et al. provides a summary of the US experience in 2020 with pharmacological EUA approvals during the COVID-19 pandemic. It also provides a description of, and clinical rationale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Project Bioshield Act
The Project Bioshield Act was an act passed by the United States Congress in 2004 calling for $5 billion for purchasing vaccines that would be used in the event of a bioterrorist attack. This was a ten-year program to acquire medical countermeasures to biological, chemical, radiological, and nuclear agents for civilian use. A key element of the Act was to allow stockpiling and distribution of vaccines which had not been tested for safety or efficacy in humans, due to ethical concerns. Efficacy of such agents cannot be directly tested in humans without also exposing humans to the chemical, biological, or radioactive threat being treated, so testing follows the FDA Animal Rule for pivotal animal efficacy. Since the 2001 terrorist attacks, the United States government has allocated nearly $50 billion to address the threat of biological weapons. U.S. funding for bioweapons-related activities focuses primarily on research for and acquisition of medicines for defense. Funding a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Remdesivir
Remdesivir, sold under the brand name Veklury, Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID‑19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in numerous countries. Remdesivir was originally developed to treat hepatitis C, and was subsequently investigated for Ebola virus disease and Marburg virus infections before being studied as a post-infection treatment for COVID‑19. Remdesivir is a prodrug that is intended to allow intracellular delivery of GS-441524 monophosphate and subsequent biotransformation into GS-441524 triphosphate, a ribonucleotide analogue inhibitor of viral RNA polymerase. The most common side effect in healthy volunteers is raised blood levels of liver enzymes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Testing
COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2. The two main types of tests detect either the presence of the virus or antibodies produced in response to infection. Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests (serology immunoassays) instead show whether someone once had the disease. They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection. It is used to assess disease prevalence, which aids the estimation of the infection fatality rate. Individual jurisdictions have adopted varied testing protocols, including whom to test, how often to test, analysis protocols, sample collection and the uses of test results. This variation has likely significantly impacted reported statistics, including case and test numbers, case fatality ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by a virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first known case was COVID-19 pandemic in Hubei, identified in Wuhan, China, in December 2019. The disease quickly spread worldwide, resulting in the COVID-19 pandemic. The symptoms of COVID‑19 are variable but often include fever, cough, headache, fatigue, breathing difficulties, Anosmia, loss of smell, and Ageusia, loss of taste. Symptoms may begin one to fourteen days incubation period, after exposure to the virus. At least a third of people who are infected Asymptomatic, do not develop noticeable symptoms. Of those who develop symptoms noticeable enough to be classified as patients, most (81%) develop mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, Hypoxia (medical), hypoxia, or more than 50% lung involvement on imaging), and 5% develop critical symptoms (respiratory failure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called the human coronavirus 2019 (HCoV-19 or hCoV-19). First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020. SARS‑CoV‑2 is a positive-sense single-stranded RNA virus that is contagious in humans. SARS‑CoV‑2 is a virus of the species ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV), related to the SARS-CoV-1 virus that caused the 2002–2004 SARS outbreak. Despite its close relation to SARS-CoV-1, its closest known relatives, with which it forms a sister group, are the derived SARS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Pandemic
The COVID-19 pandemic, also known as the coronavirus pandemic, is an ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel virus was first identified in an outbreak in the Chinese city of Wuhan in December 2019. Attempts to contain it there failed, allowing the virus to spread to other areas of Asia and later worldwide. The World Health Organization (WHO) declared the outbreak a public health emergency of international concern on 30 January 2020, and a pandemic on 11 March 2020. As of , the pandemic had caused more than cases and confirmed deaths, making it one of the deadliest in history. COVID-19 symptoms range from undetectable to deadly, but most commonly include fever, dry cough, and fatigue. Severe illness is more likely in elderly patients and those with certain underlying medical conditions. COVID-19 transmits when people breathe in air contaminated by droplets and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vitro Diagnostics
A medical test is a medical procedure performed to detect, diagnose, or monitor diseases, disease processes, susceptibility, or to determine a course of treatment. Medical tests such as, physical and visual exams, diagnostic imaging, genetic testing, chemical and cellular analysis, relating to clinical chemistry and molecular diagnostics, are typically performed in a medical setting. Types of tests By purpose Medical tests can be classified by their purposes, the most common of which are diagnosis, screening and evaluation. Diagnostic A diagnostic test is a procedure performed to confirm or determine the presence of disease in an individual suspected of having a disease, usually following the report of symptoms, or based on other medical test results. This includes posthumous diagnosis. Examples of such tests are: * Using nuclear medicine to examine a patient suspected of having a lymphoma. * Measuring the blood sugar in a person suspected of having diabetes mellitus after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
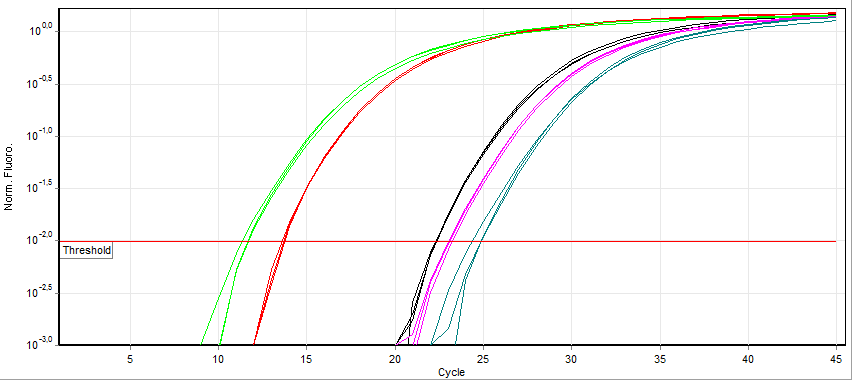
Quantitative PCR
A real-time polymerase chain reaction (real-time PCR, or qPCR) is a laboratory technique of molecular biology based on the polymerase chain reaction (PCR). It monitors the amplification of a targeted DNA molecule during the PCR (i.e., in real time), not at its end, as in conventional PCR. Real-time PCR can be used quantitatively (quantitative real-time PCR) and semi-quantitatively (i.e., above/below a certain amount of DNA molecules) (semi-quantitative real-time PCR). Two common methods for the detection of PCR products in real-time PCR are (1) non-specific fluorescent dyes that intercalate with any double-stranded DNA and (2) sequence-specific DNA probes consisting of oligonucleotides that are labelled with a fluorescent reporter, which permits detection only after hybridization of the probe with its complementary sequence. The Minimum Information for Publication of Quantitative Real-Time PCR Experiments ( MIQE) guidelines propose that the abbreviation ''qPCR'' be used for q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiviral Drug
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic (also termed antibacterial), antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees. Medical uses Most of the antiviral drugs now available are designed to help deal with HIV, herpes viruses, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2009 Swine Flu Pandemic
The 2009 swine flu pandemic, caused by the H1N1 influenza virus and declared by the World Health Organization (WHO) from June 2009 to August 2010, is the third recent flu pandemic involving the H1N1 virus (the first being the 1918–1920 Spanish flu pandemic and the second being the 1977 Russian flu). The first two cases were discovered independently in the United States in April 2009. The virus appeared to be a new strain of H1N1 that resulted from a previous triple reassortment of bird, swine, and human flu viruses which further combined with a Eurasian pig flu virus, leading to the term "swine flu". Some studies estimated that the real number of cases including asymptomatic and mild cases could be 700 million to 1.4 billion people—or 11 to 21 percent of the global population of 6.8 billion at the time. The lower value of 700 million is more than the 500 million people estimated to have been infected by the Spanish flu pandemic. However, the Spanish flu infected approxima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Centers For Disease Control And Prevention
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georgia. The agency's main goal is the protection of public health and safety through the control and prevention of disease, injury, and disability in the US and worldwide. The CDC focuses national attention on developing and applying disease control and prevention. It especially focuses its attention on infectious disease, food borne pathogens, environmental health, occupational safety and health, health promotion, injury prevention and educational activities designed to improve the health of United States citizens. The CDC also conducts research and provides information on non-infectious diseases, such as obesity and diabetes, and is a founding member of the International Association of National Public Health Institutes. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)



.jpg)

