|
Ethylene Glycol Dimethyl Ether
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water. Production Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide: :CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3 Applications as solvent and ligand left, 144px, Structure of the coordination complex NbCl3(dimethoxyethane)(3-hexyne).{{cite journal , doi=10.1021/ja8100837, title=New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism, year=2009, last1=Arteaga-Müller, first1=Rocío, last2=Tsurugi, first2=Hayato, last3=Saito, first3=Teruhiko, last4=Yanagawa, first4=Masao, last5=Oda, first5=Seiji, last6=Mashima, first6=Kazushi, journal=Journal of the American Chemical Society, volume=131, issue=15, pages ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycol Ethers
Glycol ethers are a class of chemical compounds consisting of alkyl ethers that are based on glycols such as ethylene glycol or propylene glycol. They are commonly used as solvents in paints and cleaners. They have good solvent properties while having higher boiling points than the lower-molecular-weight ethers and alcohols. The name "Cellosolve" was registered in 1924 as a United States trademark by Carbide & Carbon Chemicals Corporation (a division of Union Carbide Corporation) for "Solvents for Gums, Resins, Cellulose Esters, and the Like". "Ethyl Cellosolve" or simply "Cellosolve" consists mainly of ethylene glycol monoethyl ether and was introduced as a lower-cost solvenet alternative to ethyl lactate. "Butyl Cellosolve" (ethylene glycol monobutyl ether) was introduced in 1928, and "Methyl Cellosolve" ( ethylene glycol monomethyl ether) in 1929. Glycol ethers are designated "E-series" for or "P-series" for those made from ethylene oxide or propylene oxide, respectively. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides, or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligosaccharides
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sugars). Oligosaccharides can have many functions including cell recognition and cell adhesion. They are normally present as glycans: oligosaccharide chains are linked to lipids or to compatible amino acid side chains in proteins, by ''N''- or ''O''-glycosidic bonds. ''N''-Linked oligosaccharides are always pentasaccharides attached to asparagine via a beta linkage to the amine nitrogen of the side chain.. Alternately, ''O''-linked oligosaccharides are generally attached to threonine or serine on the alcohol group of the side chain. Not all natural oligosaccharides occur as components of glycoproteins or glycolipids. Some, such as the raffinose series, occur as storage or transport carbohydrates in plants. Others, such as maltodextrins or c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
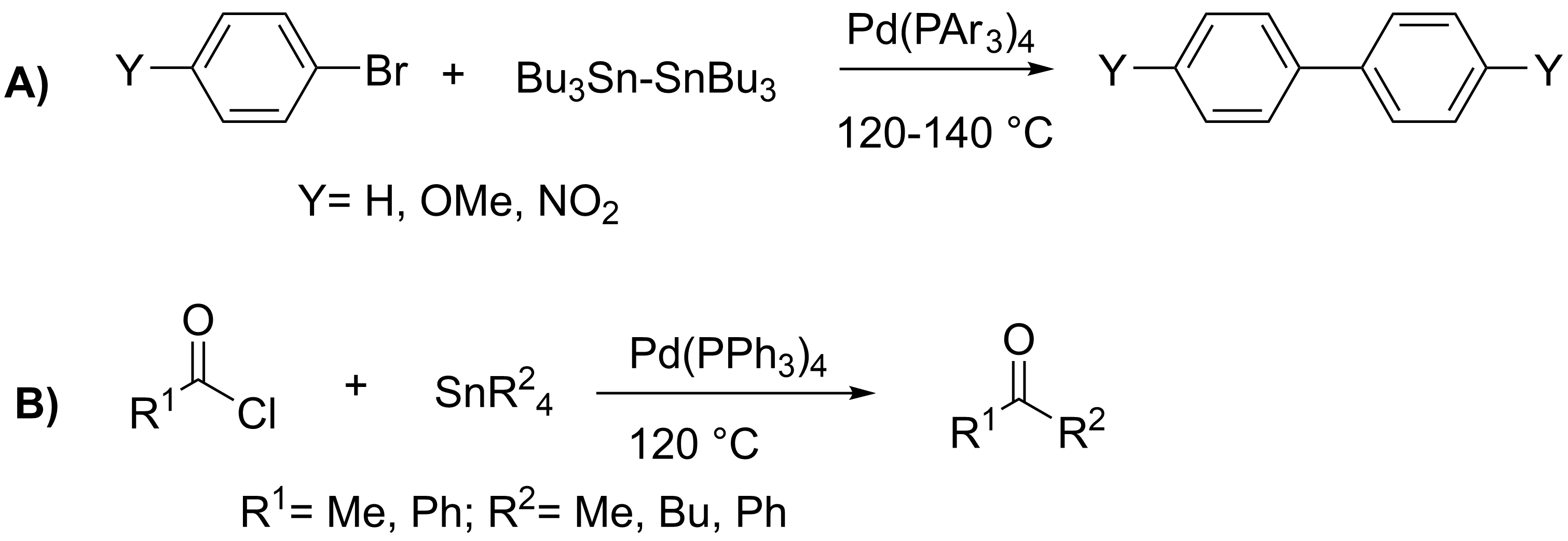
Stille Coupling
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.Hartwig, J. F. ''Organotransition Metal Chemistry, from Bonding to Catalysis''; University Science Books: New York, 2010. Stille, J. K. '' Angew. Chem. Int. Ed. Engl.'' 1986, ''25'', 508–524.ReviewFarina, V.; Krishnamurthy, V.; Scott, W. J. ''Org. React.'' 1998, ''50'', 1–652.Review : + \ \ce \ \overbrace^ + \!-\! :*\!,\ : Allyl, alkenyl, aryl, benzyl,acyl :*: halides (Cl, Br, I), pseudohalides (, OPO(OR)2), OAc The R1 group attached to the trialkyltin is normally sp2-hybridized, including vinyl, and aryl groups. These organostannanes are also stable to both air and moisture, and many of these reagents either are com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suzuki Reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize poly olefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base. Reaction mechanism The mechanism of the Suzuki r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium-catalyzed Reactions
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subsequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductions With Metal Alkoxyaluminium Hydrides
Reductions with metal alkoxyaluminium hydrides are chemical reactions that involve either the net hydrogenation of an unsaturated compound or the replacement of a reducible functional group with hydrogen by metal alkoxyaluminium hydride reagents. Introduction Sodium borohydride and lithium aluminium hydride are commonly used for the reduction of organic compounds. These two reagents are on the extremes of reactivity—whereas lithium aluminium hydride reacts with nearly all reducible functional groups, sodium borohydride reacts with a much more limited range of functional groups. Diminished or enhanced reactivity may be realized by the replacement of one or more of the hydrogens in these reagents with alkoxy groups. Additionally, substitution of hydrogen for chiral alkoxy groups in these reagents enables asymmetric reductions. Although methods involving stoichiometric amounts of chiral metal hydrides have been supplanted in modern times by enantioselective catalytic reductions, they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reaction
The Grignard reaction () is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides ( Grignard reagent) is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is ''not'' a Grignard reaction, but provides a Grignard reagent. : Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France), who published it in 1900 and was awarded the 1912 Nobel Prize in Chemistry for this work. Reaction mechanism Because carbon is more electronegative than magnesium, the carbon attached to magnesium functions as a nucleophile and attacks the electrophilic carbon atom that is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state. Based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |









4-3D-balls.png)