Suzuki reaction on:
[Wikipedia]
[Google]
[Amazon]
The Suzuki reaction is an organic reaction, classified as a 
 The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of an trialkylborane (BR3) and alkoxide (−OR); this species could be considered as being more
The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of an trialkylborane (BR3) and alkoxide (−OR); this species could be considered as being more
 Oxidative addition proceeds with retention of
Oxidative addition proceeds with retention of 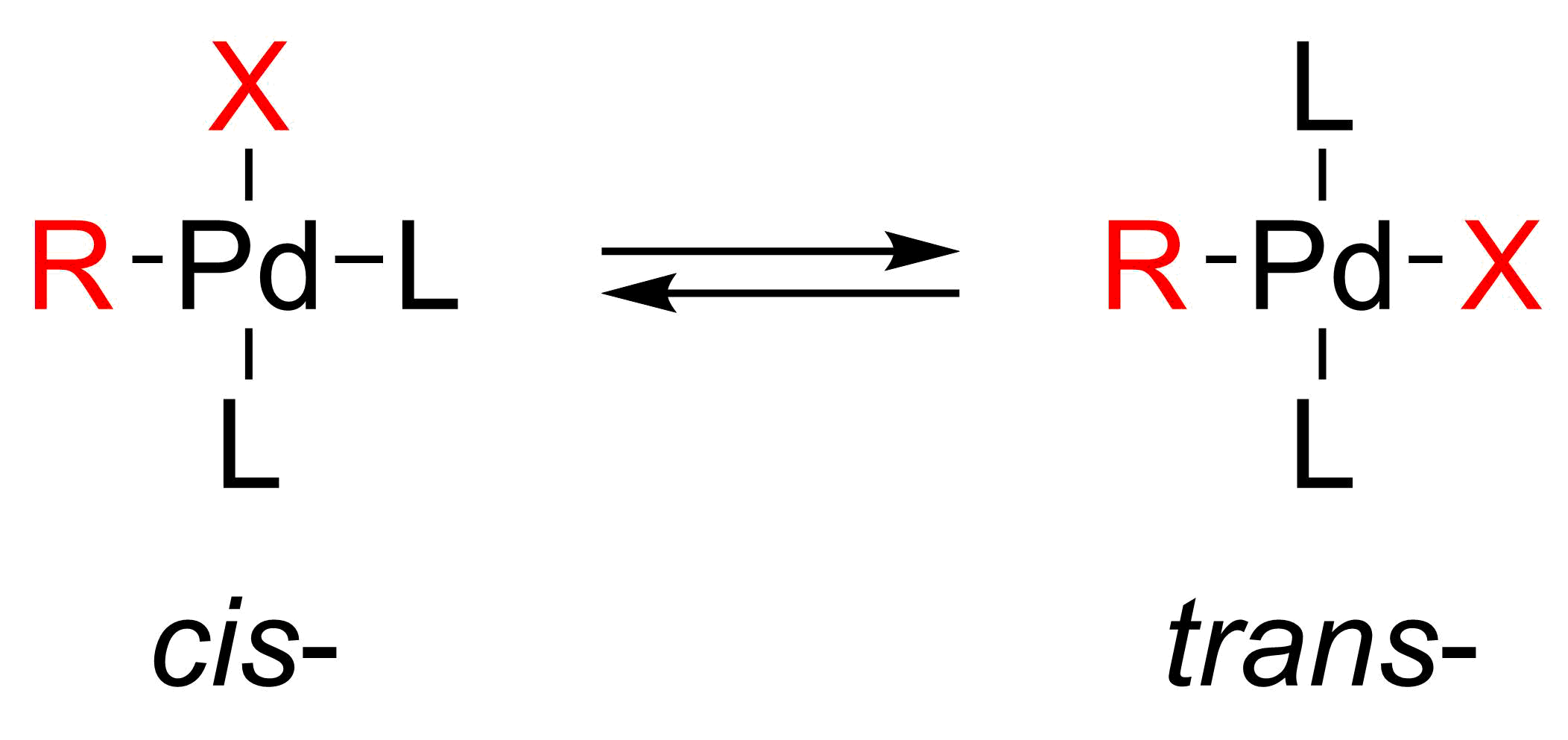 The Suzuki Coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.
The Suzuki Coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.


 The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, N-heterocyclic carbenes ligand has recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. N-Heterocyclic carbenes are more electron rich and more bulkier than the phosphine ligand. Therefore, both the steric and electronic factors of the N-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, N-heterocyclic carbenes ligand has recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. N-Heterocyclic carbenes are more electron rich and more bulkier than the phosphine ligand. Therefore, both the steric and electronic factors of the N-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
 Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom hetereogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom hetereogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
 Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the
Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the 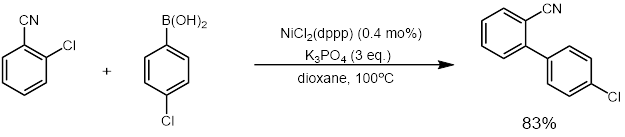 It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel
It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel  Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
 The synthesis of the
The synthesis of the 

Suzuki coupling
{{Authority control Carbon-carbon bond forming reactions Palladium Substitution reactions Name reactions
cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
, where the coupling partners are a boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
and an organohalide
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlori ...
and the catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
with Richard F. Heck and Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negi ...
for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize poly olefins, styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
s, and substituted biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
s. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling a halide (R1-X) with an organoboron
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
Organoboron ...
species (R2-BY2) using a palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
catalyst and a base.

Reaction mechanism
Themechanism
Mechanism may refer to:
* Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that ...
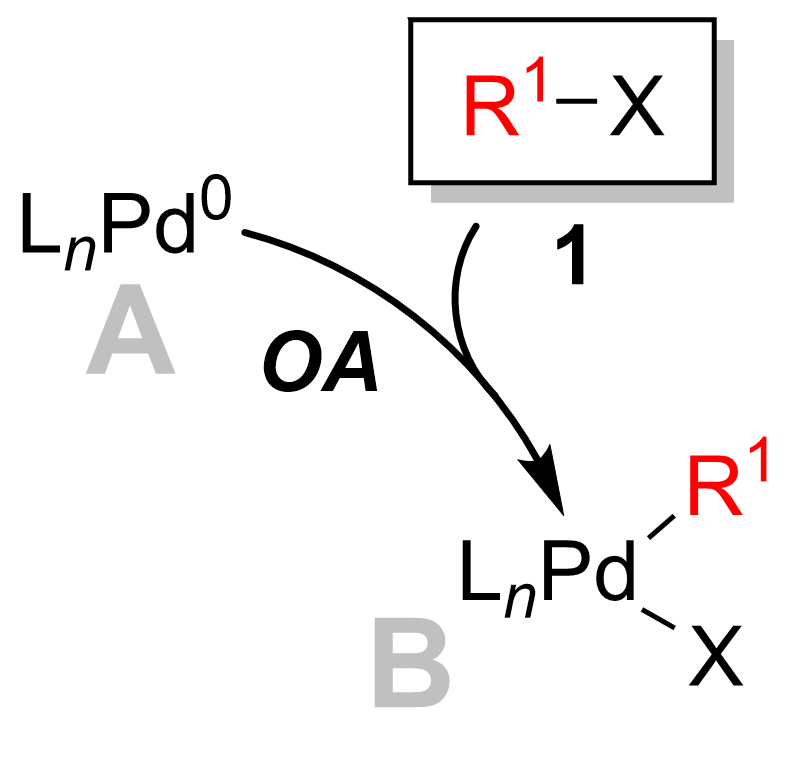
of the Suzuki reaction is best viewed from the perspective of the palladium catalyst. The catalytic cycle is initiated by the formation of an active Pd0 catyltic species, A. This participates in the oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
of palladium to the halide reagent 1 to form the organopalladium Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves t ...
intermediate B. Reaction ( metathesis) with base gives intermediate C, which via transmetalation with the boron-ate complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valence i ...
D (produced by reaction of the boronic acid reagent 2 with base) forms the transient organopalladium Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves t ...
species E. Reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
step leads to the formation of the desired product 3 and restores the original palladium catalyst A which completes the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
.
 The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of an trialkylborane (BR3) and alkoxide (−OR); this species could be considered as being more
The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of an trialkylborane (BR3) and alkoxide (−OR); this species could be considered as being more nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
and then more reactive towards the palladium complex present in the transmetalation step. Duc and coworkers investigated the role of the base in the reaction mechanism for the Suzuki coupling and they found that the base has three roles: Formation of the palladium complex rPd(OR)L2 formation of the trialkyl borate and the acceleration of the reductive elimination step by reaction of the alkoxide with the palladium complex.
Oxidative addition
In most cases the oxidative addition is therate determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
of the catalytic cycle. During this step, the palladium catalyst is oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
from palladium(0) to palladium(II). The catalytically active palladium species A is coupled with the aryl halide substrate 1 to yield an organopalladium complex B. As seen in the diagram below, the oxidative addition step breaks the carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
-halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
bond where the palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
is now bound to both the halogen (X) as well as the R1 group.
 Oxidative addition proceeds with retention of
Oxidative addition proceeds with retention of stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
with vinyl halide
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl ...
s, while giving inversion
Inversion or inversions may refer to:
Arts
* , a French gay magazine (1924/1925)
* ''Inversion'' (artwork), a 2005 temporary sculpture in Houston, Texas
* Inversion (music), a term with various meanings in music theory and musical set theory
* ...
of stereochemistry with allylic and benzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a subst ...
halides. The oxidative addition initially forms the cis
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonome ...
–palladium complex, which rapidly isomerizes to the trans-complex.
 The Suzuki Coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.
The Suzuki Coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.

Transmetalation
Transmetalation is anorganometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
reaction where ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
are transferred from one species to another. In the case of the Suzuki coupling the ligands are transferred from the organoboron species D to the palladium(II) complex C where the base that was added in the prior step is exchanged with the R2 substituent on the organoboron species to give the new palladium(II) complex E. The exact mechanism of transmetalation for the Suzuki coupling remains to be discovered. The organoboron compounds do not undergo transmetalation in the absence of base and it is therefore widely believed that the role of the base is to activate the organoboron compound as well as facilitate the formation of R1-Pdll-O''t''Bu intermediate (C) from oxidative addition product R1-Pdll-X (B).

Reductive elimination
The final step is the reductive elimination step where the palladium(II) complex (E) eliminates the product (3) and regenerates the palladium(0) catalyst(A). Usingdeuterium labelling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specif ...
, Ridgway ''et al.'' have shown the reductive elimination proceeds with retention of stereochemistry.
 The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, N-heterocyclic carbenes ligand has recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. N-Heterocyclic carbenes are more electron rich and more bulkier than the phosphine ligand. Therefore, both the steric and electronic factors of the N-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, N-heterocyclic carbenes ligand has recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. N-Heterocyclic carbenes are more electron rich and more bulkier than the phosphine ligand. Therefore, both the steric and electronic factors of the N-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
Advantages
The advantages of Suzuki coupling over other similar reactions include availability of common boronic acids, mild reaction conditions, and its less toxic nature.Boronic acids
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
are less toxic and safer for the environment than organotin and organozinc compound
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compoun ...
s. It is easy to remove the inorganic by-products from the reaction mixture. Further, this reaction is preferable because it uses relatively cheap and easily prepared reagents. Being able to use water as a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
makes this reaction more economical, eco-friendly, and practical to use with a variety of water-soluble reagents. A wide variety of reagents can be used for the Suzuki coupling, e.g., aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
- or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
-boronic acids and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
- or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
-halides. Work has also extended the scope of the reaction to incorporate alkyl bromides. In addition to many different type of halides being possible for the Suzuki coupling reaction, the reaction also works with pseudohalide
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic ...
s such as triflate
In organic chemistry, triflate ( systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ...
s (OTf), as replacements for halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
. The relative reactivity for the coupling partner with the halide or pseudohalide is: R2–I > R2–OTf > R2–Br >> R2–Cl. Boronic ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
s and organotrifluoroborate salts may be used instead of boronic acids. The catalyst can also be a palladium nanomaterial-based catalyst Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalyst ...
. With a novel organophosphine Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphine ...
ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
( SPhos), a catalyst loading of down to 0.001 mol% has been reported:. These advances and the overall flexibility of the process have made the Suzuki coupling widely accepted for chemical synthesis.
Applications
Industrial applications
The Suzuki coupling reaction is scalable and cost-effective for use in the synthesis of intermediates forpharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and rel ...
or fine chemicals
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used f ...
. The Suzuki reaction was once limited by high levels of catalyst and the limited availability of boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
s. Replacements for halides were also found, increasing the number of coupling partners for the halide or pseudohalide as well. Scaled up reactions have been carried out in the synthesis of a number of important biological compounds such as CI-1034 which used a triflate
In organic chemistry, triflate ( systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ...
and boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
coupling partners which was run on an 80 kilogram scale with a 95% yield.
Another example is the coupling of 3-pyridylborane and 1-bromo-3-(methylsulfonyl)benzene that formed an intermediate that was used in the synthesis of a potential central nervous system agent. The coupling reaction to form the intermediate produced (278 kilograms) in a 92.5% yield.
 Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom hetereogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom hetereogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
Synthetic applications
The Suzuki coupling has been frequently used in syntheses of complex compounds. The Suzuki coupling has been used on acitronellal
Citronellal or rhodinal ( C10 H18 O) is a monoterpenoid aldehyde, the main component in the mixture of terpenoid chemical compounds that give citronella oil its distinctive lemon scent.
Citronellal is a main isolate in distilled oils from the p ...
derivative for the synthesis of caparratriene, a natural product that is highly active against leukemia:
Variations
Metal catalyst
Various catalytic uses of metals other than palladium (especially nickel) have been developed. The first nickel catalyzed cross-coupling reaction was reported by Percec and co-workers in 1995 using aryl mesylates and boronic acids. Even though a higher amount of nickelcatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
was needed for the reaction, around 5 mol %, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
is not as expensive or as precious a metal as palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
. The nickel catalyzed Suzuki coupling reaction also allowed a number of compounds that did not work or worked worse for the palladium catalyzed system than the nickel-catalyzed system. The use of nickel catalysts has allowed for electrophiles that proved challenging for the original Suzuki coupling using palladium, including substrates such as phenols, aryl ethers, esters, phosphates, and fluorides.
 Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the
Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the cross-coupling
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
, using triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
(PPh3) instead of the more expensive ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
previously used. However, the nickel-catalyzed cross-coupling still required high catalyst loadings (3-10%), required excess ligand (1-5 equivalents) and remained sensitive to air and moisture. Advancements by Han and co-workers have tried to address that problem by developing a method using low amounts of nickel catalyst (<1 mol%) and no additional equivalents of ligand.
 It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel
It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 1 ...
catalyst (G3DenP-Ni) that was made from dendrimer
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The ...
s.
 Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
Amide coupling
Nickel catalysis can construct C-C bonds from amides. Despite the inherently inert nature of amides as synthons, the following methodology can be used to prepare C-C bonds. The coupling procedure is mild and tolerant of myriad functional groups, including: amines, ketones, heterocycles, groups with acidic protons. This technique can also be used to prepare bioactive molecules and to unite heterocycles in controlled ways through shrewd sequential cross-couplings. A general review of the reaction scheme is given below. The synthesis of the
The synthesis of the tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoske ...
binding compound (antiproliferative
Cytostasis (cyto – cell; stasis – stoppage) is the inhibition of cell growth and multiplication. Cytostatic refers to a cellular component or medicine that inhibits cell division.
Cytostasis is an important prerequisite for structured multic ...
agent) was carried out using trimethoxyamide and a heterocyclic pinacolatoboron coupling partner on a gram scale.

Organoboranes
Arylboronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
s are comparatively cheaper than other organoboranes and a wide variety of aryl boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
s are commercially available. Hence, it has been widely used in Suzuki reaction as an organoborane partner. Aryltrifluoroborate salts are another class of organoboranes that are frequently used because they are less prone to protodeboronation compared to aryl boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
s. They are easy to synthesize and can be easily purified. Aryltrifluoroborate salts can be formed from boronic acids
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
by the treatment with potassium hydrogen fluoride
Potassium bifluoride is the inorganic compound with the chemical formula, formula . This colourless salt consists of the potassium cation () and the bifluoride anion (). The salt is used as an Glass etching, etchant for glass. Sodium bifluoride is ...
which can then be used in the Suzuki coupling reaction.

Solvent variations
The Suzuki coupling reaction is different from other coupling reactions in that it can be run in biphasic organic-water, water-only, or no solvent. This increased the scope of coupling reactions, as a variety of water-soluble bases, catalyst systems, and reagents could be used without concern over their solubility in organic solvent. Use of water as a solvent system is also attractive because of the economic and safety advantages. Frequently used in solvent systems for Suzuki coupling aretoluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) a ...
, THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
, dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
, and DMF. The most frequently used bases are K2CO3, KO''t''Bu, Cs2CO3, K3PO4, NaOH
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
, and NEt3.
See also
* Chan-Lam coupling *Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a sub ...
* Hiyama coupling
* Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ...
* Negishi coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) specie ...
* Petasis reaction
The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.
Reported in 1993 by Nicos Petasis ...
* Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or v ...
* Stille reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electro ...
* List of organic reactions
Well-known reactions and reagents in organic chemistry include
0-9
* 1,2-Wittig rearrangement
* 1,3-Dipolar cycloaddition
* 2,3-Wittig rearrangement
A
* Abramovitch–Shapiro tryptamine synthesis
* Acetalisation
* Acetoacetic ester condensat ...
References
External links
*Suzuki coupling
{{Authority control Carbon-carbon bond forming reactions Palladium Substitution reactions Name reactions