|
Electron Scattering Experiment
Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors. The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks. Electrons may be scattered through a solid in several ways: *Not at all: no electron scattering occurs at all and the beam passes straight through. *Single scattering: when an electron is scattered just once. *Pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
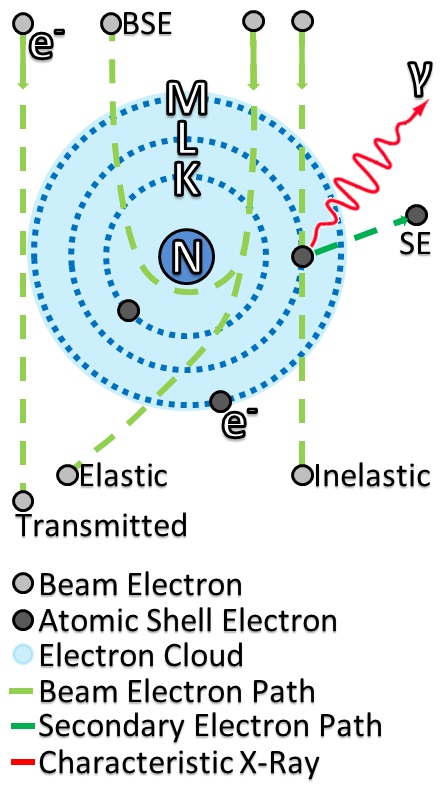
Electron-beam Interaction And Transmission With Sample
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode (the electrode connected to the negative terminal of the voltage supply). They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein ''Kathodenstrahlen'', or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the ''electron''. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen. Description Cathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. To release electrons into the tube, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
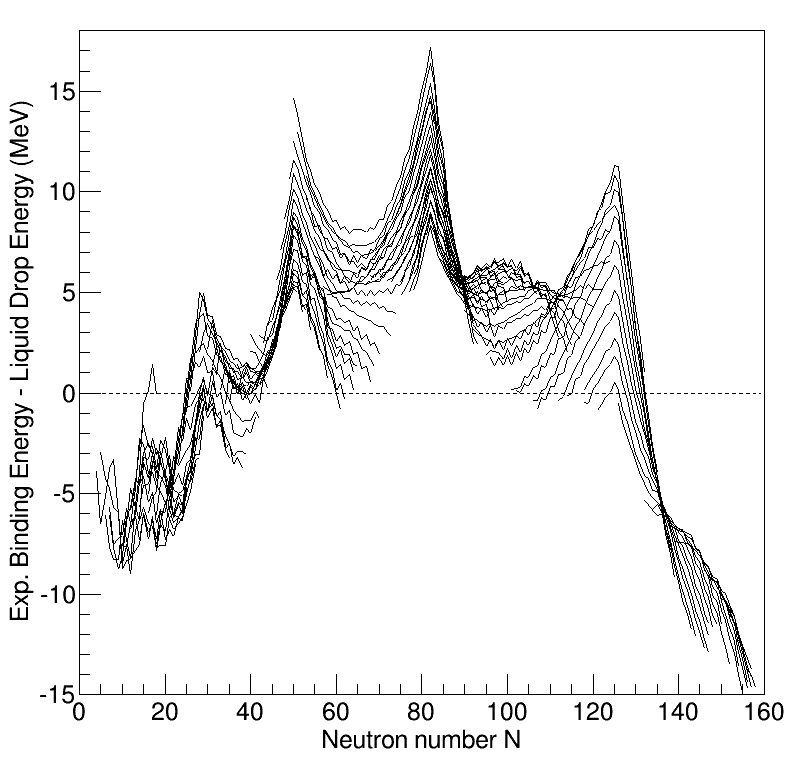
Nuclear Structure
Understanding the structure of the atomic nucleus is one of the central challenges in nuclear physics. Models The liquid drop model The liquid drop model is one of the first models of nuclear structure, proposed by Carl Friedrich von Weizsäcker in 1935. It describes the nucleus as a semiclassical fluid made up of neutrons and protons, with an internal repulsive electrostatic force proportional to the number of protons. The quantum mechanical nature of these particles appears via the Pauli exclusion principle, which states that no two nucleons of the same kind can be at the same state. Thus the fluid is actually what is known as a Fermi liquid. In this model, the binding energy of a nucleus with Z protons and N neutrons is given by :E_ = a_ A - a_ A^ - a_ \frac - a_ \frac - \delta(A,Z) where A=Z+N is the total number of nucleons (Mass Number). The terms proportional to A and A^ represent the volume and surface energy of the liquid drop, the term proportional to Z^ represents t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hadron
In particle physics, a hadron (; grc, ἁδρός, hadrós; "stout, thick") is a composite subatomic particle made of two or more quarks held together by the strong interaction. They are analogous to molecules that are held together by the electric force. Most of the mass of ordinary matter comes from two hadrons: the proton and the neutron, while most of the mass of the protons and neutrons is in turn due to the binding energy of their constituent quarks, due to the strong force. Hadrons are categorized into two broad families: baryons, made of an odd number of quarks (usually three quarks) and mesons, made of an even number of quarks (usually two quarks: one quark and one antiquark). Protons and neutrons (which make the majority of the mass of an atom) are examples of baryons; pions are an example of a meson. "Exotic" hadrons, containing more than three valence quarks, have been discovered in recent years. A tetraquark state (an exotic meson), named the Z(4430), was discove ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic Force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventionally called ''electrostatic force'' or Coulomb force. Although the law was known earlier, it was first published in 1785 by French physicist Charles-Augustin de Coulomb, hence the name. Coulomb's law was essential to the development of the theory of electromagnetism, maybe even its starting point, as it made it possible to discuss the quantity of electric charge in a meaningful way. The law states that the magnitude of the electrostatic force of attraction or repulsion between two point charges is directly proportional to the product of the magnitudes of charges and inversely proportional to the square of the distance between them. Coulomb studied the repulsive force between bodies having electrical charges of the same sign: Coulomb also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trajectory
A trajectory or flight path is the path that an object with mass in motion follows through space as a function of time. In classical mechanics, a trajectory is defined by Hamiltonian mechanics via canonical coordinates; hence, a complete trajectory is defined by position and momentum, simultaneously. The mass might be a projectile or a satellite. For example, it can be an orbit — the path of a planet, asteroid, or comet as it travels around a central mass. In control theory, a trajectory is a time-ordered set of states of a dynamical system (see e.g. Poincaré map). In discrete mathematics, a trajectory is a sequence (f^k(x))_ of values calculated by the iterated application of a mapping f to an element x of its source. Physics of trajectories A familiar example of a trajectory is the path of a projectile, such as a thrown ball or rock. In a significantly simplified model, the object moves only under the influence of a uniform gravitational force field. This can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thomson Scattering
Thomson scattering is the elastic scattering of electromagnetic radiation by a free charged particle, as described by classical electromagnetism. It is the low-energy limit of Compton scattering: the particle's kinetic energy and photon frequency do not change as a result of the scattering. This limit is valid as long as the photon energy is much smaller than the mass energy of the particle: \nu\ll mc^2/h , or equivalently, if the wavelength of the light is much greater than the Compton wavelength of the particle (e.g., for electrons, longer wavelengths than hard x-rays). Description of the phenomenon In the low-energy limit, the electric field of the incident wave (photon) accelerates the charged particle, causing it, in turn, to emit radiation at the same frequency as the incident wave, and thus the wave is scattered. Thomson scattering is an important phenomenon in plasma physics and was first explained by the physicist J. J. Thomson. As long as the motion of the particle is no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
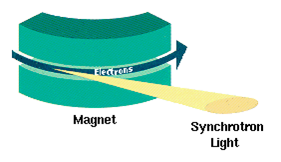
Synchrotron Emission
Synchrotron radiation (also known as magnetobremsstrahlung radiation) is the electromagnetic radiation emitted when relativistic charged particles are subject to an acceleration perpendicular to their velocity (). It is produced artificially in some types of particle accelerators, or naturally by fast electrons moving through magnetic fields. The radiation produced in this way has a characteristic polarization and the frequencies generated can range over a large portion of the electromagnetic spectrum. Synchrotron radiation is similar to bremsstrahlung radiation, which is emitted by a charged particle when the acceleration is parallel to the direction of motion. The general term for radiation emitted by particles in a magnetic field is ''gyromagnetic radiation'', for which synchrotron radiation is the ultra-relativistic special case. Radiation emitted by charged particles moving non-relativistically in a magnetic field is called cyclotron emission. For particles in the mildly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deep Inelastic Scattering
Deep inelastic scattering is the name given to a process used to probe the insides of hadrons (particularly the baryons, such as protons and neutrons), using electrons, muons and neutrinos. It provided the first convincing evidence of the reality of quarks, which up until that point had been considered by many to be a purely mathematical phenomenon. It is a relatively new process, first attempted in the 1960s and 1970s. It is an extension of Rutherford scattering to much higher energies of the scattering particle and thus to much finer resolution of the components of the nuclei. Henry Way Kendall, Jerome Isaac Friedman and Richard E. Taylor were joint recipients of the Nobel Prize of 1990 "for their pioneering investigations concerning deep inelastic scattering of electrons on protons and bound neutrons, which have been of essential importance for the development of the quark model in particle physics." Description To explain each part of the terminology, "scattering" refers to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bremsstrahlung
''Bremsstrahlung'' (), from "to brake" and "radiation"; i.e., "braking radiation" or "deceleration radiation", is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into radiation (i.e., photons), thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. ''Bremsstrahlung'' has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the decelerated particles increases. Broadly speaking, ''bremsstrahlung'' or braking radiation is any radiation produced due to the deceleration (negative acceleration) of a charged particle, which includes synchrotron radiation (i.e., photon emission by a relativistic particle), cyclotron radiation (i.e. photon emission by a non ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bhabha Scattering
In quantum electrodynamics, Bhabha scattering is the electron-positron scattering process: ::e^+ e^- \rightarrow e^+ e^- There are two leading-order Feynman diagrams contributing to this interaction: an annihilation process and a scattering process. Bhabha scattering is named after the Indian physicist Homi J. Bhabha. The Bhabha scattering rate is used as a luminosity monitor in electron-positron colliders. Differential cross section To leading order, the spin-averaged differential cross section for this process is ::\frac = \frac \left( u^2 \left( \frac + \frac \right)^2 + \left( \frac \right)^2 + \left( \frac \right)^2 \right) \, where ''s'',''t'', and ''u'' are the Mandelstam variables, \alpha is the fine-structure constant, and \theta is the scattering angle. This cross section is calculated neglecting the electron mass relative to the collision energy and including only the contribution from photon exchange. This is a valid approximation at collision energies small com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mott Scattering
Mott scattering in physics, also referred to as spin-coupling inelastic Rutherford scattering, Coulomb scattering, is the separation of the two spin states of an electron beam by scattering the beam off the Coulomb field of heavy atoms. It is named after Nevill Francis Mott, who first developed the theory. It is mostly used to measure the spin polarization of an electron beam. In lay terms, Mott scattering is similar to Rutherford Scattering, Rutherford scattering but electrons are used instead of alpha particles as they do not interact via the strong force (only weak and electromagnetic). This enables them to penetrate the atomic nucleus, giving valuable insight into the nuclear structure. The electrons are often fired at gold foil because gold has a high atomic number (Z), is non-reactive (does not form an oxide layer), and can be easily made into a thin film (reducing multiple scattering). The presence of a spin-orbit term in the scattering potential introduces a spin dependen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |