|
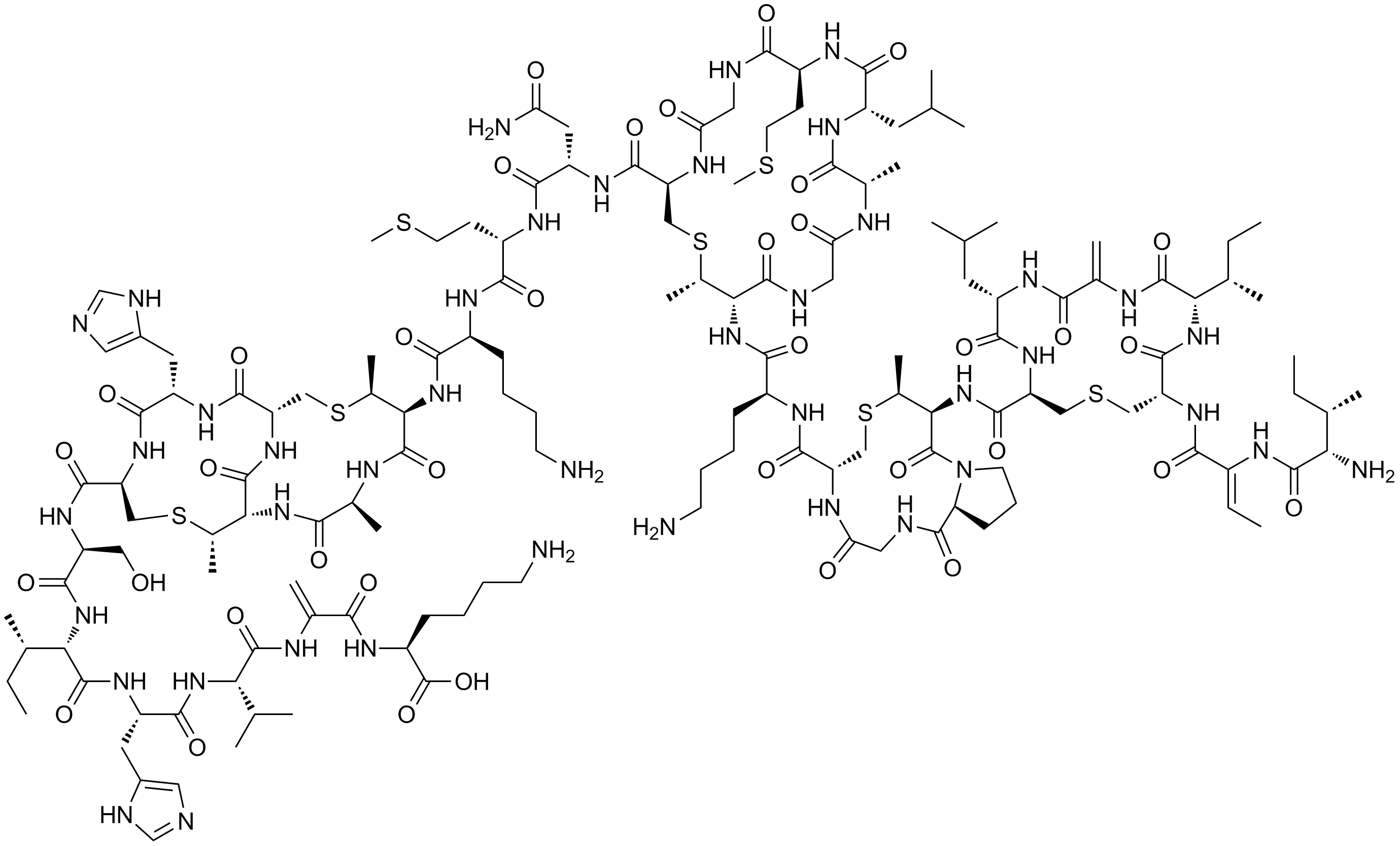
Dehydroamino Acid
In biochemistry, a dehydroamino acid is an amino acids, usually with a C=C double bond in its side chain. Dehydroamino acids are not coded by DNA, but arise via post-transcriptional modification. Examples A common dehydroamino acid is dehydroalanine, which otherwise exists only as a residue in proteins and peptides. The dehydroalanine residue is obtained dehydration of serine-containing protein/peptide (alternatively, removal of H2S from cysteine). Another example is dehydrobutyrine, derived from dehydration of threonine. Generally, amino acid residues are unreactive toward nucleophiles, but the dehydroamino acids are exceptions to this pattern. For example, dehydroalanine adds cysteine and lysine to form covalent crosslinks. An unusual dehydroamino acid is dehydroglycine (DHG) because it does not contain a carbon-carbon double bond. Instead it is the imine of glyoxalic acid. It arises by the radical-induced degradation of tyrosine. N-Acyl derivatives Dehydroamino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells,Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function.Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenomena.As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-transcriptional Modification
Transcriptional modification or co-transcriptional modification is a set of biological processes common to most eukaryotic cells by which an RNA primary transcript is chemically altered following transcription from a gene to produce a mature, functional RNA molecule that can then leave the nucleus and perform any of a variety of different functions in the cell. There are many types of post-transcriptional modifications achieved through a diverse class of molecular mechanisms. One example is the conversion of precursor messenger RNA transcripts into mature messenger RNA that is subsequently capable of being translated into protein. This process includes three major steps that significantly modify the chemical structure of the RNA molecule: the addition of a 5' cap, the addition of a 3' polyadenylated tail, and RNA splicing. Such processing is vital for the correct translation of eukaryotic genomes because the initial precursor mRNA produced by transcription often contains both exo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
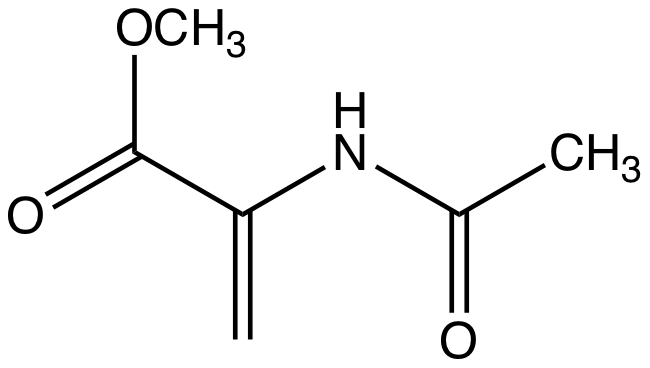
Dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As an amino acid residue, it is unusual because it has an unsaturated backbone. Structure and reactivity Like most primary enamines, dehydroalanine is unstable. Dehydroalanine hydrolyze to pyruvate. ''N''-Acylated derivatives of dehydroalanine, such as peptides and related compounds, are stable. For example, methyl 2-acetamidoacrylate is the N-acetylated derivative of the ester. As a residue in a peptide, it is generated by a post translational modification. The required precursors are serine or cysteine residues, which undergo enzyme-mediated loss of water and hydrogen sulfide, respectively. Most amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine or some other dehydroamino acids are exceptions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
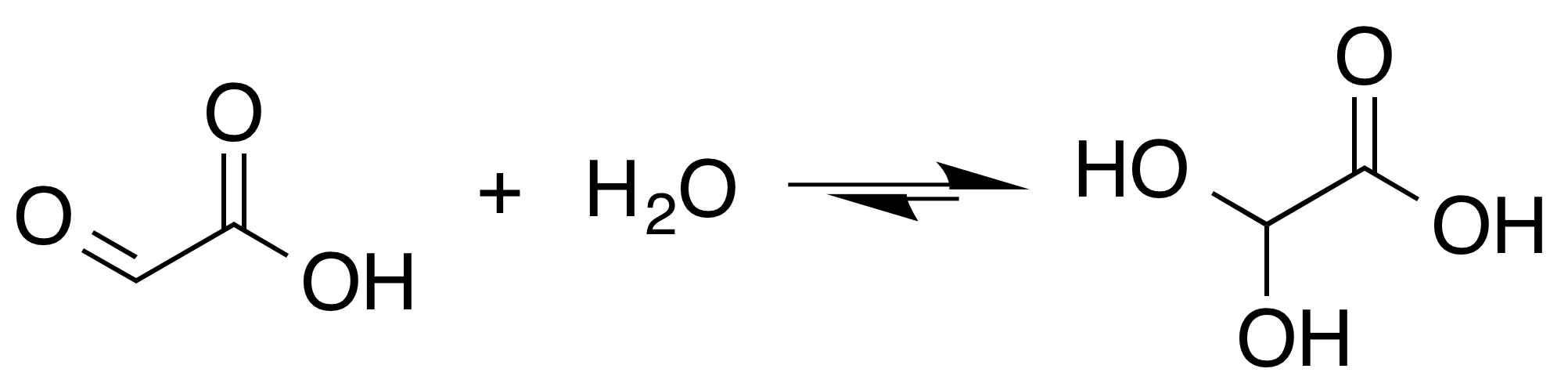
Glyoxalic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of Hydrogen atom, hydrogen to a target (substrate) molecule with three-dimensional Enantioselective synthesis, spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information (what chemists refer to as chirality) to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
) , image = Nobel Prize.png , alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "MDCCCXXXIII" above, followed by (smaller) "OB•" then "MDCCCXCVI" below. , awarded_for = Outstanding contributions in chemistry , presenter = Royal Swedish Academy of Sciences , location = Stockholm, Sweden , reward = 9 million SEK (2017) , year = 1901 , holder = Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless (2022) , most_awards = Frederick Sanger and Karl Barry Sharpless (2) , website nobelprize.org, previous = 2021 , year2=2022, main=2022, next=2023 The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |