|
Cadmium Chloride
Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and CdCl2•5H2O. Structure Cadmium chloride forms a layered structure consisting of octahedral Cd2+ centers linked with chloride ligands. Cadmium iodide, CdI2, has a similar structure, but the iodide ions are arranged in a HCP lattice, whereas in CdCl2 the chloride ions are arranged in a CCP lattice.N. N. Greenwood, A. Earnshaw, ''Chemistry of the Elements'', 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997. Chemical properties Cadmium chloride dissolves well in water and other polar solvents. It is a mild Lewis acid. :CdCl2 + 2 Cl− → dCl4sup>2− Solutions of equimolar cadmium chloride and potassium chloride give potassium cadmium tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the Interna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fragments. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocadmium Compound
An organocadmium compound is an organometallic compound containing a carbon to cadmium chemical bond. Organocadmium chemistry describes physical properties, synthesis, reactions and use of these compounds. Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common. The synthetic utility of organocadmium compounds is limited. The simplest organocadmium compound is dimethylcadmium. It is a linear molecule with a C-Cd bond length of 213 pm. Organocadmium compounds are typically sensitive to air, light, and moisture. Synthesis Dimethylcadmium and diethylcadmium were reported in 1917 by Erich Krause. In general, they are prepared by transmetalation or by an exchange reaction between an alkylating agent and a cadmium salt. According to one procedure, diethylcadmium is produced the reaction of cadmium bromide with two equivalents of the Grignard reagent ethylmagnesium bromide in diethyl ether. Diethylcadmium is a colorless oil with melti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compounds. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli. Economic impact In 2006, around 7.4 million tons of inorganic, organic, and special pigments were marketed worldwide. Estimated at around US$14.86 billion in 2018 and will rise at over 4.9% CAGR from 2019 to 2026. The global demand for pigments was roughly US$20.5 billion in 2009. According to an April 2018 report by '' Bloomberg Businessweek'', the estimated value of the pigment industry globally is $30 billion. The value of titanium dioxide – used to enhance the white brightness of many products – was placed at $13.2 billion per year, while the color Ferrari red is valued at $300 million each year. Physical princip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Yellow
Cadmium pigments are a class of pigments that contain cadmium. Most of the cadmium produced worldwide has been for use in rechargeable nickel–cadmium batteries, which have been replaced by other rechargeable nickel-chemistry cell varieties such as NiMH cells, but about half of the remaining consumption of cadmium, which is approximately annually, is used to produce colored cadmium pigments. The principal pigments are a family of yellow, orange and red cadmium sulfides and sulfoselenides, as well as compounds with other metals. Cadmium is toxic to humans and other animals in very small amounts, especially when it is inhaled, which often happens when working with powdered pigment or breathing the dust from chalk pastels. As a result, it is not appropriate for children to use any art supplies that contain cadmium pigments. However, because the pigments have some desirable qualities, such as resistance to fading, some adult artists continue to use them. Artists' paints ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid.Egon Wiberg, Arnold Frederick Holleman (2001''Inorganic Chemistry'' Elsevier It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century. Production Cadmium sulfide can be prepared by the precipitation from soluble cadmium(II) salts with sulfide ion. This reaction has been used for gravimetric analysis and qualitative inorganic analysis.The preparative route and the subsequent treatment of the product, affects the polymorphic form that is produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac ( ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method for producing hydrochloric acid, as perhaps manifested in the following recipe from his ("The Book of Secrets"): However, it appears that in most of his experiments al-Razi disregarded the gaseous products, concentrating instead on the color c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
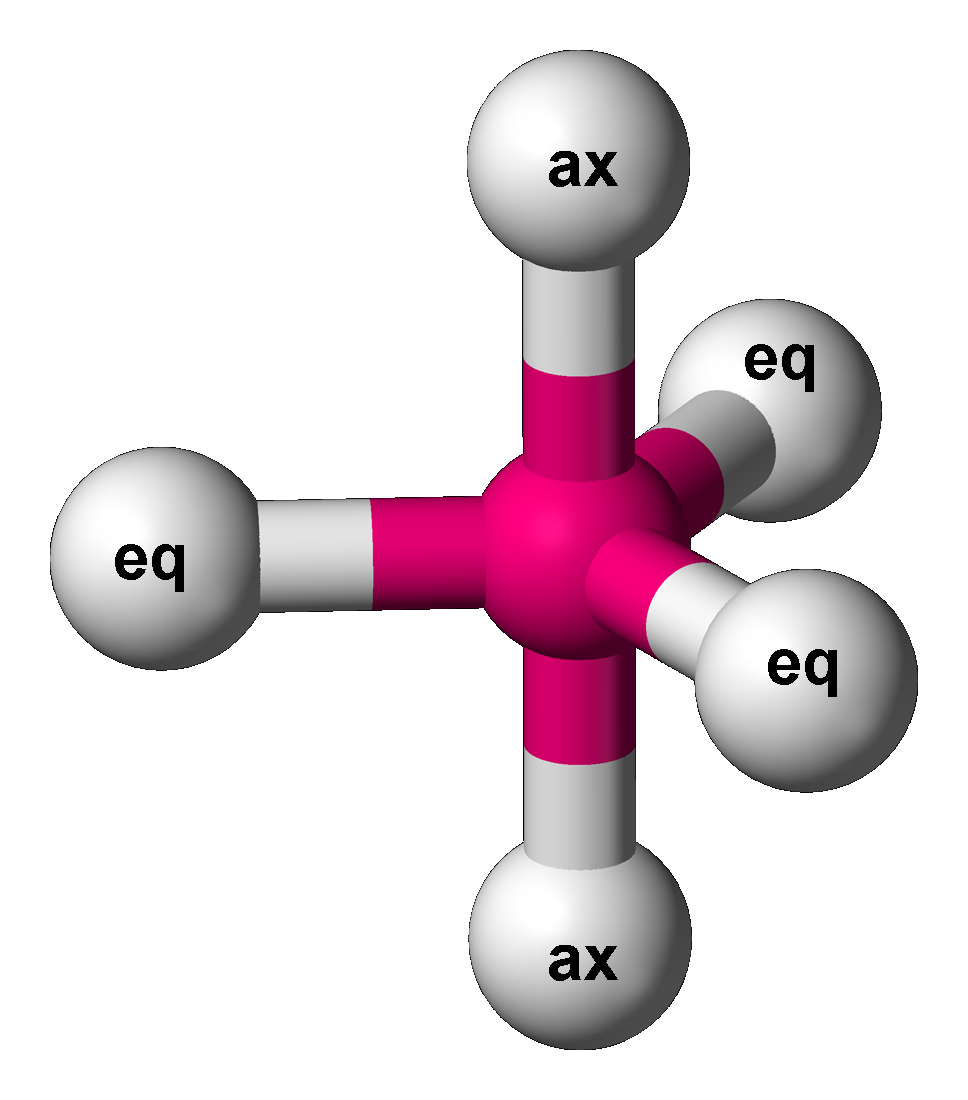
Trigonal Bipyramid Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Close Packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a lattice packing is :\frac \approx 0.74048. The same packing density can also be achieved by alternate stackings of the same close-packed planes of spheres, including structures that are aperiodic in the stacking direction. The Kepler conjecture states that this is the highest density that can be achieved by any arrangement of spheres, either regular or irregular. This conjecture was proven by T. C. Hales. Highest density is known only for 1, 2, 3, 8, and 24 dimensions. Many crystal structures are based on a close-packing of a single kind of atom, or a close-packing of large ions with smaller ions filling the spaces between them. The cubic and hexagonal arrangements are very close to one anoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |