|
Coupled Substitution
Coupled substitution is the geological process by which two elements simultaneous substitute into a crystal in order to maintain overall electrical neutrality and keep the charge constant. In forming a solid solution series, ionic size is more important than ionic charge, as this can be compensated for elsewhere in the structure. Ionic size To make a geometrically stable structure in a mineral, atoms must fit together in terms of both their size and charge. The atoms have to fit together so that their electron shells can interact with one another and they also have to produce a neutral molecule. For these reasons the sizes and electron shell structure of atoms determine what element combinations are possible and the geometrical form that various minerals take. Because electrons are donated and received, it is the ionic radius of the element that controls the size and determines how atoms fit together in minerals. Examples * Coupled substitutions are common in the silicate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feldspar Series
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust, and 41% of the Earth's continental crust by weight. Feldspars crystalize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Compositions The feldspar group of minerals consists of tectosilicates, silicate minerals in which silicon ions are linked by shared oxygen ions to form a three-dimensional network. Compositions of major elements in common feldspars can be expressed in terms of three endmembers: * potassium feldspar (K-spar) en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bityite
Bityite is considered a rare mineral, and it is an endmember to the margarite mica sub-group found within the phyllosilicate group. The mineral was first described by Antoine François Alfred Lacroix in 1908, and later its chemical composition was concluded by Professor Hugo Strunz classification, Strunz.Strunz, H. (1956) Bityit, ein berylliumglimmer. Zeitschrift für Kristallographie, 107, 325-330. Bityite has a close association with beryl, and it generally crystallizes in pseudomorphs after it, or in cavities associated with reformed beryl crystals.Lahti, S. I. and Saikkonen, R. (1985) Bityite 2M1 from Eräjärvi compared with related Li-Be brittle micas. Bulletin of the Geological Society of Finland, 57, 207-215. The mineral is considered a late-stage constituent in lithium bearing pegmatites,Lin, J-C. and Guggenheim, S. (1983) The crystal structure of a Li,Be-rich brittle mica: a dioctaheral-trioctahedral intermediate. American Mineralogist, 68, 130-142. and has only been enc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hornblende
Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rocks. The general formula is . Physical properties Hornblende has a hardness of 5–6, a specific gravity of 3.0 to 3.6, and is typically an opaque green, dark green, brown, or black color. It tends to form slender prismatic to bladed crystals, diamond-shaped in cross-section, or is present as irregular grains or fibrous masses. Its planes of cleavage intersect at 56° and 124° angles. Hornblende is most often confused with the pyroxene series and biotite mica, which are also dark minerals found in granite and charnockite. Pyroxenes differ in their cleavage planes, which intersect at 87° and 93°. Hornblende is an inosilicate (chain silicate) mineral, built around double chains of silica tetrahedra. These chains extend the length ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edenite
Edenite is a double chain silicate mineral of the amphibole group with the general chemical composition NaCa2Mg5(Si7Al)O22(OH)2. Edenite is named for the locality of Edenville, Orange County, New York, where it was first described. Occurrence Edenite has been found primarily in metamorphic rocks, occurring in pods of other magnesium rich minerals within a marble formation or with garnet rich lherzolites from deep within the Earth's crust. Thus, finding edenite in the field can indicate high temperature regional metamorphism of the surrounding rocks. Uses and importance While edenite is not important for commercial or industrial applications, it is often studied because of its unique chemical substitution properties. Results from research performed on amphiboles have shown that edenite is particularly suited for fitting chloride anions into its chemical framework. This makes edenite a good candidate for use in chlorine isotope fractionation in amphibole-bearing rocks. Many s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tremolite
Tremolite is a member of the amphibole group of silicate minerals with composition: Ca2(Mg5.0-4.5Fe2+0.0-0.5)Si8O22(OH)2. Tremolite forms by metamorphism of sediments rich in dolomite and quartz. Tremolite forms a series with actinolite and ferro-actinolite. Pure magnesium tremolite is creamy white, but the color grades to dark green with increasing iron content. It has a hardness on Mohs scale of 5 to 6. Nephrite, one of the two minerals of the gemstone jade, is a green variety of tremolite. The fibrous form of tremolite is one of the six recognised types of asbestos. This material is toxic, and inhaling the fibers can lead to asbestosis, lung cancer and both pleural and peritoneal mesothelioma. Fibrous tremolite is sometimes found as a contaminant in vermiculite, chrysotile (itself a type of asbestos) and talc. Occurrence Tremolite is an indicator of metamorphic grade since at high temperatures it converts to diopside. Tremolite occurs as a result of contact metam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphibole
Amphibole () is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups. Mineralogy Amphiboles crystallize into two crystal systems, monoclinic and orthorhombic. In chemical composition and general characteristics they are similar to the pyroxenes. The chief differences from pyroxenes are that (i) amphiboles contain essential hydroxyl (OH) or halogen (F, Cl) and (ii) the basic structure is a double chain of tetrahedra (as opposed to the single chain structure of pyroxene). Most apparent, in hand specimens, is that amphiboles form oblique cleavage planes (at around 120 degrees), whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinel Group
The spinels are any of a class of minerals of general formulation which crystallise in the cubic (isometric) crystal system, with the X anions (typically chalcogens, like oxygen and sulfur) arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice.H-J MeyerFestkörperchemiein: H-J Meyer (ed.), ''Riedel Moderne Anorganische Chemie'', Walter de Gruyter, 2012, . Retrieved 15 April 2018. Although the charges of A and B in the prototypical spinel structure are +2 and +3, respectively (), other combinations incorporating divalent, trivalent, or tetravalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon, are also possible. The anion is normally oxygen; when other chalcogenides constitute the anion sublattice the structure is referred to as a thiospinel. A and B can also be the same metal with different valences, as is the case with magnetite, (as ), wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
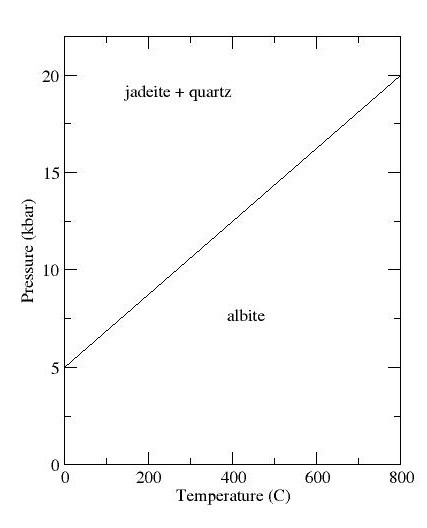
Jadeite Sodium Aluminum Silicate Burma 3025
Jadeite is a pyroxene mineral with composition Na Al Si2 O6. It is hard (Mohs hardness of about 6.5 to 7.0), very tough, and dense, with a specific gravity of about 3.4. It is found in a wide range of colors, but is most often found in shades of green or white. Jadeite is formed only in subduction zones on continental margins, where rock undergoes metamorphism at high pressure but relatively low temperature. Jadeite is the principal mineral making up the most valuable form of jade, a precious stone particularly prized in China. Most gem-quality jadeite jade comes from northern Myanmar. Jade tools and implements have been found at Stone Age sites, showing that the mineral has been prized by humans since before the beginning of written history. Name The name ''jadeite'' is derived (via french: jade and la, ilia) from the Spanish phrase "piedra de ijada" which means "stone of the side". The Latin version of the name, ''lapis nephriticus'', is the origin of the term nephrite, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diopside Aoste
Diopside is a monoclinic pyroxene mineral with composition . It forms complete solid solution series with hedenbergite () and augite, and partial solid solutions with orthopyroxene and pigeonite. It forms variably colored, but typically dull green crystals in the monoclinic prismatic class. It has two distinct prismatic cleavages at 87 and 93° typical of the pyroxene series. It has a Mohs hardness of six, a Vickers hardness of 7.7 GPa at a load of 0.98 N, and a specific gravity of 3.25 to 3.55. It is transparent to translucent with Refractive index, indices of refraction of nα=1.663–1.699, nβ=1.671–1.705, and nγ=1.693–1.728. The optic angle is 58° to 63°. Formation Diopside is found in ultramafic (kimberlite and peridotite) igneous rock (geology), rocks, and diopside-rich augite is common in mafic rocks, such as olivine basalt and andesite. Diopside is also found in a variety of metamorphic rock, metamorphic rocks, such as in contact metamorphosed skarns developed f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jadeite
Jadeite is a pyroxene mineral with composition Na Al Si2 O6. It is hard (Mohs hardness of about 6.5 to 7.0), very tough, and dense, with a specific gravity of about 3.4. It is found in a wide range of colors, but is most often found in shades of green or white. Jadeite is formed only in subduction zones on continental margins, where rock undergoes metamorphism at high pressure but relatively low temperature. Jadeite is the principal mineral making up the most valuable form of jade, a precious stone particularly prized in China. Most gem-quality jadeite jade comes from northern Myanmar. Jade tools and implements have been found at Stone Age sites, showing that the mineral has been prized by humans since before the beginning of written history. Name The name ''jadeite'' is derived (via french: jade and la, ilia) from the Spanish phrase "piedra de ijada" which means "stone of the side". The Latin version of the name, ''lapis nephriticus'', is the origin of the term nephrite, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diopside
Diopside is a monoclinic pyroxene mineral with composition . It forms complete solid solution series with hedenbergite () and augite, and partial solid solutions with orthopyroxene and pigeonite. It forms variably colored, but typically dull green crystals in the monoclinic prismatic class. It has two distinct prismatic cleavages at 87 and 93° typical of the pyroxene series. It has a Mohs hardness of six, a Vickers hardness of 7.7 GPa at a load of 0.98 N, and a specific gravity of 3.25 to 3.55. It is transparent to translucent with indices of refraction of nα=1.663–1.699, nβ=1.671–1.705, and nγ=1.693–1.728. The optic angle is 58° to 63°. Formation Diopside is found in ultramafic ( kimberlite and peridotite) igneous rocks, and diopside-rich augite is common in mafic rocks, such as olivine basalt and andesite. Diopside is also found in a variety of metamorphic rocks, such as in contact metamorphosed skarns developed from high silica dolomites. It is an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |