|
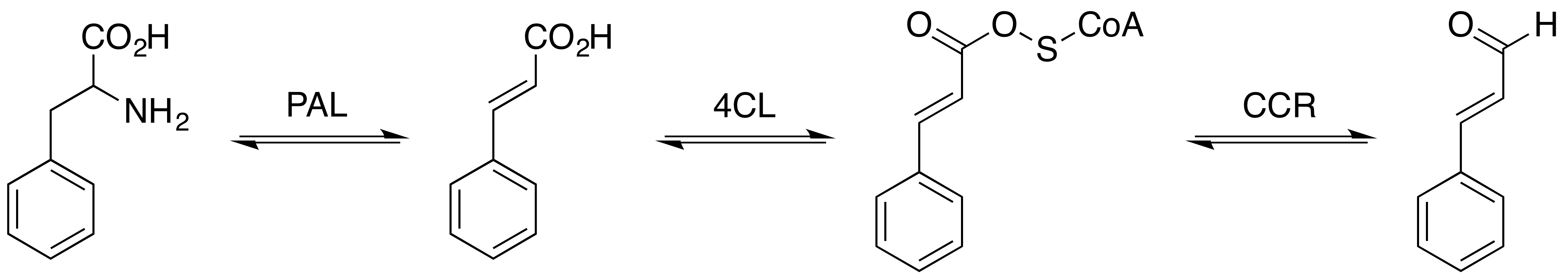
Coniferaldehyde
Coniferyl aldehyde is an organic compound with the formula HO(CH3O)C6H3CH=CHCHO. It is a derivative of cinnamaldehyde, featuring 4-hydroxy and 3-methoxy substituents. It is a major precursor to lignin. Biosynthetic role In sweetgum (''Liquidambar styraciflua''), coniferyl aldehyde is a precursor to sinapaldehyde via hydroxylation mediated by coniferyl aldehyde 5-hydroxylase. Coniferyl aldehyde is reduced to coniferyl alcohol by the action of dehydrogenase enzymes. It is found in ''Senra incana'' (Hibisceae). It is a low molecular weight phenol that is susceptible to extraction from cork stoppers into wine.Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. Elvira Conde, Estrella CadahĂa, MarĂa ConcepciĂłn GarcĂa-Vallejo and BrĂgida Fernández de SimĂłn, J. Agric. Food Chem., 1998, volume 46, pp 3166–3171 See also * Phenolic compounds in wine The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenolsâ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus ''Cinnamomum''. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''trans''-cinnamaldehyde. The molecule consists of a benzene rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as an electron-donating group, but as an electron-withdrawing group if at the ''meta'' position. At the ''ortho'' position, steric effects are likely to cause a significant alteration in the Hammett equation prediction which otherwise follows the same trend as that of the ''para'' position. Occurrence The simplest of methoxy compounds are methanol and dimethyl ether. Other methoxy ethers include anisole and vanillin. Many alkoxides contain methoxy groups, e.g. tetramethyl orthosilicate and titanium methoxide. Such compounds are often classified as methoxides. Esters with a methoxy group can be referred to as methyl esters, and the —COOCH3 substituent is called a methoxycarbonyl. Biosynthesis In nature, methoxy groups are found on nucleosi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors. History Lignin was first mentioned in 1813 by the Swiss botanist A. P. de Candolle, who described it as a fibrous, tasteless material, insoluble in water and alcohol but soluble in weak alkaline solutions, and which can be precipitated from solution using acid. He named the substance “lignine”, which is derived from the Latin word '' lignum'', meaning wood. It is one of the most abundant organic polymers on Earth, exceeded only by cellulose. Lignin constitutes 30% of non-fossil organic carbon on Earth, and 20 to 35% of the dry mass of wood. Lignin is present in red algae, which suggest that the common ancestor of plants and red algae ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sinapaldehyde
Sinapaldehyde is an organic compound with the formula HO(CH3O)2C6H2CH=CHCHO. It is a derivative of cinnamaldehyde, featuring one hydroxy group and two methoxy groups as substituents. It is an intermediate in the formation of sinapyl alcohol, a lignol that is a major precursor to lignin. Biosynthetic role In sweetgum (''Liquidambar styraciflua''), sinapaldehyde arises in two steps from coniferyl aldehyde beginning with hydroxylation mediated by coniferyl aldehyde 5-hydroxylase. The diphenol is then methylated at the 5-OH by the action of caffeate ''O''-methyltransferase. Sinapaldehyde is reduced to the alcohol by the action of dehydrogenase enzymes. In ''Arabidopsis thaliana'', the enzyme dihydroflavonol 4-reductase uses NADP+ to reduce sinapaldehyde to sinapyl alcohol. It is found in ''Senra incana'' (Hibisceae). It is a low molecular weight phenol that is susceptible to extraction from cork stoppers into wine.Polyphenolic Composition of ''Quercus suber'' Cork from Different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation
In chemistry, hydroxylation can refer to: *(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound. *(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a molecule. The ''pattern of hydroxylation'' refers to the location of hydroxy groups on a molecule or material. Hydroxylation reactions Synthetic hydroxylations Installing hydroxyl groups into organic compounds can be effected by various metal catalysts. Many such catalysts are biomimetic, i.e. they are inspired by or intended to mimic enzymes such as cytochrome P450. Whereas many hydroxylations insert O atoms into bonds, some reactions ''add'' OH groups to unsaturated substrates. The Sharpless dihydroxylation is such a reaction: it converts alkenes into diols. The hydroxy groups are provided by hydrogen peroxide, which adds across the double bond of alkenes. Biological hydroxylation In biochemistry, hydroxylation reactions are often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydrogenase
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde. IUBMB Classification Oxidoreductases, enzymes that catalyze oxidation-reduction reactions, constitute Class EC 1 of the IUBMB classification of enzyme-catalyzed reactions. Any of these may be called dehydrogenases, especially those in which NAD+ is the electron acceptor (oxidant), but reductase is also used when the physiological emphasis on reduction of the substrate, and oxidase is used ''only'' when O2 is the electron acceptor. The systematic name of an oxidoreductase is "donor:acceptor oxidore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Senra Incana
''Senra incana'' is a flowering plant species in the genus '' Senra''. The plant produces the phenolic compounds coniferaldehyde, scopoletin, sinapaldehyde and syringaldehyde Syringaldehyde is an organic compound that occurs in trace amounts widely in nature. Some species of insects use syringaldehyde in their chemical communication systems. ''Scolytus multistriatus'' uses it as a signal to find a host tree during ovip ...Pharmacologically active phenylpropanoids from Senra incana. Farah MH, Samuelsson G. Planta Med. 1992 Feb;58(1):14-8. References External links ''Senra incana'' on JStor Hibisceae Plants described in 1786 Taxa named by Antonio José Cavanilles {{Hibisceae-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Phenol
In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants. Classification Various classification schemes can be applied. A commonly used scheme is based on the number of carbons and was devised by Jeffrey Harborne and Simmonds in 1964 and published in 1980: C6-C7-C6 Diarylheptanoids are not included in this Harborne classification. They can also be classified on the basis of their number of phenol groups. They can therefore be called ''simple phenols'' or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cork (material)
Cork is an Permeability (earth sciences), impermeable buoyancy, buoyant material, the Cork cambium, phellem layer of bark (botany), bark tissue that is harvested for commercial use primarily from ''Quercus suber'' (the cork oak), which is native to southwest Europe and northwest Africa. Cork is composed of suberin, a hydrophobic substance. Because of its impermeable, buoyant, elastic, and fire retardant properties, it is used in a variety of products, the most common of which is wine stoppers. The Dehesa (pastoral management), montado landscape of Portugal produces approximately half of the cork harvested annually worldwide, with Corticeira Amorim being the leading company in the industry. Cork was examined microscopically by Robert Hooke, which led to his discovery and naming of the cell (biology), cell. Cork composition varies depending on Geography, geographic origin, climate and soil conditions, Genetics, genetic origin, tree dimensions, age (virgin or reproduction), and gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolic Compounds In Wine
The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers ( proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids. Origin of the phenolic compounds The natural phenols are not evenly distributed within the fruit. Phenolic acids are largely present in the pulp, anthocyanins and stilbenoids in the skin, and other phenols (catechins, proanthocyanidins and flavonols) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |