|
Sinapaldehyde
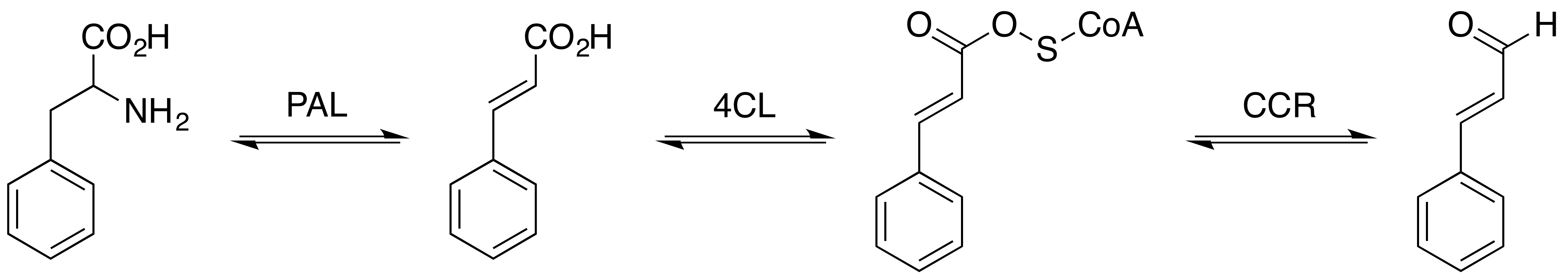
Sinapaldehyde is an organic compound with the formula HO(CH3O)2C6H2CH=CHCHO. It is a derivative of cinnamaldehyde, featuring one hydroxy group and two methoxy groups as substituents. It is an intermediate in the formation of sinapyl alcohol, a lignol that is a major precursor to lignin. Biosynthetic role In sweetgum (''Liquidambar styraciflua''), sinapaldehyde arises in two steps from coniferyl aldehyde beginning with hydroxylation mediated by coniferyl aldehyde 5-hydroxylase. The diphenol is then methylated at the 5-OH by the action of caffeate ''O''-methyltransferase. Sinapaldehyde is reduced to the alcohol by the action of dehydrogenase enzymes. In ''Arabidopsis thaliana'', the enzyme dihydroflavonol 4-reductase uses NADP+ to reduce sinapaldehyde to sinapyl alcohol. It is found in '' Senra incana'' (Hibisceae). It is a low molecular weight phenol that is susceptible to extraction from cork stoppers into wine.Polyphenolic Composition of ''Quercus suber'' Cork from Differe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coniferyl Aldehyde
Coniferyl aldehyde is an organic compound with the formula HO(CH3O)C6H3CH=CHCHO. It is a derivative of cinnamaldehyde, featuring 4-hydroxy and 3-methoxy substituents. It is a major precursor to lignin. Biosynthetic role In sweetgum (''Liquidambar styraciflua''), coniferyl aldehyde is a precursor to sinapaldehyde via hydroxylation mediated by coniferyl aldehyde 5-hydroxylase. Coniferyl aldehyde is reduced to coniferyl alcohol by the action of dehydrogenase enzymes. It is found in '' Senra incana'' (Hibisceae). It is a low molecular weight phenol that is susceptible to extraction from cork stoppers into wine.Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. Elvira Conde, Estrella Cadahía, María Concepción García-Vallejo and Brígida Fernández de Simón, J. Agric. Food Chem., 1998, volume 46, pp 3166–3171 See also * Phenolic compounds in wine The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sinapyl Alcohol
Sinapyl alcohol is an organic compound structurally related to cinnamic acid. It is biosynthetized via the phenylpropanoid biochemical pathway, its immediate precursor being sinapaldehyde. This phytochemical is one of the monolignols, which are precursor to lignin or lignans. It is also a biosynthetic precursor to various stilbenoids and coumarins. See also * Sinapinic acid * Syringol * Syringaldehyde *Syringic acid *Acetosyringone * Sinapine *Canolol *Phenolic content in wine The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include ... References {{DEFAULTSORT:Sinapyl Alcohol Monolignols Ethers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Senra Incana
''Senra incana'' is a flowering plant species in the genus '' Senra''. The plant produces the phenolic compounds coniferaldehyde, scopoletin, sinapaldehyde and syringaldehyde Syringaldehyde is an organic compound that occurs in trace amounts widely in nature. Some species of insects use syringaldehyde in their chemical communication systems. ''Scolytus multistriatus'' uses it as a signal to find a host tree during ovip ...Pharmacologically active phenylpropanoids from Senra incana. Farah MH, Samuelsson G. Planta Med. 1992 Feb;58(1):14-8. References External links ''Senra incana'' on JStor Hibisceae Plants described in 1786 Taxa named by Antonio José Cavanilles {{Hibisceae-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coniferyl Aldehyde
Coniferyl aldehyde is an organic compound with the formula HO(CH3O)C6H3CH=CHCHO. It is a derivative of cinnamaldehyde, featuring 4-hydroxy and 3-methoxy substituents. It is a major precursor to lignin. Biosynthetic role In sweetgum (''Liquidambar styraciflua''), coniferyl aldehyde is a precursor to sinapaldehyde via hydroxylation mediated by coniferyl aldehyde 5-hydroxylase. Coniferyl aldehyde is reduced to coniferyl alcohol by the action of dehydrogenase enzymes. It is found in '' Senra incana'' (Hibisceae). It is a low molecular weight phenol that is susceptible to extraction from cork stoppers into wine.Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. Elvira Conde, Estrella Cadahía, María Concepción García-Vallejo and Brígida Fernández de Simón, J. Agric. Food Chem., 1998, volume 46, pp 3166–3171 See also * Phenolic compounds in wine The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroflavonol 4-reductase
In enzymology, a dihydrokaempferol 4-reductase () is an enzyme that catalyzes the chemical reaction :cis-3,4-leucopelargonidin + NADP+ \rightleftharpoons (+)- dihydrokaempferol + NADPH + H+ Thus, the two substrates of this enzyme are cis-3,4-leucopelargonidin and NADP+, whereas its 3 products are (+)-dihydrokaempferol, NADPH, and H+. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is cis-3,4-leucopelargonidin:NADP+ 4-oxidoreductase. Other names in common use include dihydroflavanol 4-reductase (DFR), dihydromyricetin reductase, NADPH-dihydromyricetin reductase, and dihydroquercetin reductase. This enzyme participates in flavonoid biosynthesis. Function Anthocyanidins, common plant pigments, are further reduced by the enzyme dihydroflavonol 4-reductase (DFR) to the corresponding colorless leucoanthocyanidins. DFR uses dihydromyricetin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Phenol
In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants. Classification Various classification schemes can be applied. A commonly used scheme is based on the number of carbons and was devised by Jeffrey Harborne and Simmonds in 1964 and published in 1980: C6-C7-C6 Diarylheptanoids are not included in this Harborne classification. They can also be classified on the basis of their number of phenol groups. They can therefore be called ''simple phenols'' o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cork (material)
Cork is an impermeable buoyant material, the phellem layer of bark tissue that is harvested for commercial use primarily from ''Quercus suber'' (the cork oak), which is native to southwest Europe and northwest Africa. Cork is composed of suberin, a hydrophobic substance. Because of its impermeable, buoyant, elastic, and fire retardant properties, it is used in a variety of products, the most common of which is wine stoppers. The montado landscape of Portugal produces approximately half of the cork harvested annually worldwide, with Corticeira Amorim being the leading company in the industry. Cork was examined microscopically by Robert Hooke, which led to his discovery and naming of the cell. Cork composition varies depending on geographic origin, climate and soil conditions, genetic origin, tree dimensions, age (virgin or reproduction), and growth conditions. However, in general, cork is made up of suberin (average of about 40%), lignin (22%), polysaccharides (cellulose an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolic Content In Wine
The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers ( proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids. Origin of the phenolic compounds The natural phenols are not evenly distributed within the fruit. Phenolic acids are largely present in the pulp, anthocyanins and stilbenoids in the skin, and other phenols (catechins, proanthocyanidins and flavon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syringaldehyde
Syringaldehyde is an organic compound that occurs in trace amounts widely in nature. Some species of insects use syringaldehyde in their chemical communication systems. ''Scolytus multistriatus'' uses it as a signal to find a host tree during oviposition. Because it contains many functional groups, it can be classified in many ways - aromatic, aldehyde, phenol. It is a colorless solid (impure samples appear yellowish) that is soluble in alcohol and polar organic solvents. Its refractive index is 1.53. Natural sources Syringaldehyde can be found naturally in the wood of spruce and maple trees. Syringaldehyde is also formed in oak barrels and extracted into whisky, which it gives spicy, smoky, hot and smoldering wood aromas. Preparation This compound may be prepared from syringol by the Duff reaction: : See also *Phenolic content in wine * Syringol *Syringic acid *Acetosyringone *Sinapyl alcohol * Sinapinic acid * Sinapaldehyde * Sinapine *Canolol Canolol is a phenoli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus ''Cinnamomum''. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''trans''-cinnamaldehyde. The molecule consists of a benz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syringic Acid
Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite. Natural occurrence Syringic acid can be found in several plants including '' Ardisia elliptica'' and ''Schumannianthus dichotomus''. Synthesis Syringic acid can be prepared by selectively hydrolyzing ( demethylating) eudesmic acid with 20% sulfuric acid. Presence in food Syringic acid can be found in several fruits including olives, dates, spices, pumpkin, grapes, acai palm, honey, red wine, among others. Its presence in the ancient Egyptian drink shedeh could confirm it was made out of grape, as syringic acid is released by the breakdown of the compound malvidin, also found in red wine. It is also found in vinegar. Applications Various studies have found syringic acid to exhibit useful pharmaceutical properties such as anti-oxidant, anti-microbial, anti-inflammation, anti-cancer, and anti-diabetic. Syringic acid can be enzymatically pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetosyringone
Acetosyringone is a phenolic natural product and a chemical compound related to acetophenone and 2,6-dimethoxyphenol. It was first described in relation to lignan/phenylpropanoid-type phytochemicals, with isolation from a variety of plant sources, in particular, in relation to wounding and other physiologic changes. Occurrence and biological role Historically, this substance has been best known for its involvement in plant-pathogen recognition, especially its role as a signal attracting and transforming unique, oncogenic bacteria in genus '' Agrobacterium''. The ''virA'' gene on the Ti plasmid of ''Agrobacterium tumefaciens'' and the Ri plasmid of '' Agrobacterium rhizogenes'' is used by these soil bacteria to infect plants, via its encoding for a receptor for acetosyringone and other phenolic phytochemicals exuded by plant wounds. This compound also allows higher transformation efficiency in plants, as shown in ''A. tumefaciens''-mediated transformation procedures, and so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |