Dehydrogenase on:
[Wikipedia]
[Google]
[Amazon]
A dehydrogenase is an
 Dehydrogenases oxidize a substrate by transferring hydrogen to an electron acceptor, common electron acceptors being NAD+ or FAD. This would be considered an oxidation of the substrate, in which the substrate either loses hydrogen atoms or gains an oxygen atom (from water). The name "dehydrogenase" is based on the idea that it facilitates the removal (de-) of hydrogen (-hydrogen-), and is an enzyme (-ase). Dehydrogenase reactions come most commonly in two forms: the transfer of a hydride and release of a proton (often with water as a second reactant), and the transfer of two hydrogens.
Dehydrogenases oxidize a substrate by transferring hydrogen to an electron acceptor, common electron acceptors being NAD+ or FAD. This would be considered an oxidation of the substrate, in which the substrate either loses hydrogen atoms or gains an oxygen atom (from water). The name "dehydrogenase" is based on the idea that it facilitates the removal (de-) of hydrogen (-hydrogen-), and is an enzyme (-ase). Dehydrogenase reactions come most commonly in two forms: the transfer of a hydride and release of a proton (often with water as a second reactant), and the transfer of two hydrogens.
 A represents the substrate that will be oxidized, while B is the hydride acceptor. Note how when the hydride is transferred from A to B, the A has taken on a positive charge; this is because the enzyme has taken two electrons from the substrate in order to reduce the acceptor to BH.
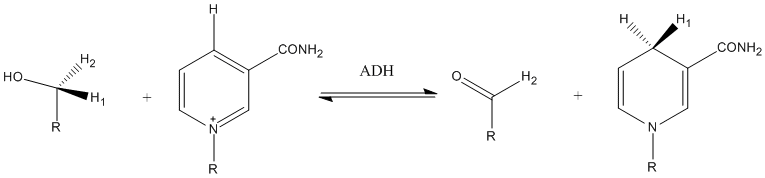
The result of a dehydrogenase catalyzed reaction is not always the acquisition of a positive charge. Sometimes the substrate loses a proton. This may leave free electrons on the substrate that move into a double bond. This happens frequently when an alcohol is the substrate; when the proton on the oxygen leaves, the free electrons on the oxygen will be used to create a double bond, as seen in the oxidation of ethanol to acetaldehyde carried out by alcohol dehydrogenase in the image on the right.
Another possibility is that a water molecule will enter the reaction, contributing a hydroxide ion to the substrate and a proton to the environment. The net result on the substrate is the addition of one oxygen atom. This is seen for example in the oxidation of acetaldehyde to
A represents the substrate that will be oxidized, while B is the hydride acceptor. Note how when the hydride is transferred from A to B, the A has taken on a positive charge; this is because the enzyme has taken two electrons from the substrate in order to reduce the acceptor to BH.
The result of a dehydrogenase catalyzed reaction is not always the acquisition of a positive charge. Sometimes the substrate loses a proton. This may leave free electrons on the substrate that move into a double bond. This happens frequently when an alcohol is the substrate; when the proton on the oxygen leaves, the free electrons on the oxygen will be used to create a double bond, as seen in the oxidation of ethanol to acetaldehyde carried out by alcohol dehydrogenase in the image on the right.
Another possibility is that a water molecule will enter the reaction, contributing a hydroxide ion to the substrate and a proton to the environment. The net result on the substrate is the addition of one oxygen atom. This is seen for example in the oxidation of acetaldehyde to
 In the above case, the dehydrogenase has transferred a hydride while releasing a proton, H+, but dehydrogenases can also transfer two hydrogens, using FAD as an electron acceptor. This would be depicted as AH2 + B ↔ A + BH2.
A double bond is normally formed in between the two atoms that the hydrogens were taken from, as in the case of
In the above case, the dehydrogenase has transferred a hydride while releasing a proton, H+, but dehydrogenases can also transfer two hydrogens, using FAD as an electron acceptor. This would be depicted as AH2 + B ↔ A + BH2.
A double bond is normally formed in between the two atoms that the hydrogens were taken from, as in the case of
 Dehydrogenase and
Dehydrogenase and
 Dehydrogenase enzymes transfer electrons from the substrate to an electron carrier; what carrier is used depends on the reaction taking place. Common electron acceptors used by this subclass are NAD+, FAD, and NADP+. Electron carriers are reduced in this process and considered oxidizers of the substrate. Electron carriers are
Dehydrogenase enzymes transfer electrons from the substrate to an electron carrier; what carrier is used depends on the reaction taking place. Common electron acceptors used by this subclass are NAD+, FAD, and NADP+. Electron carriers are reduced in this process and considered oxidizers of the substrate. Electron carriers are


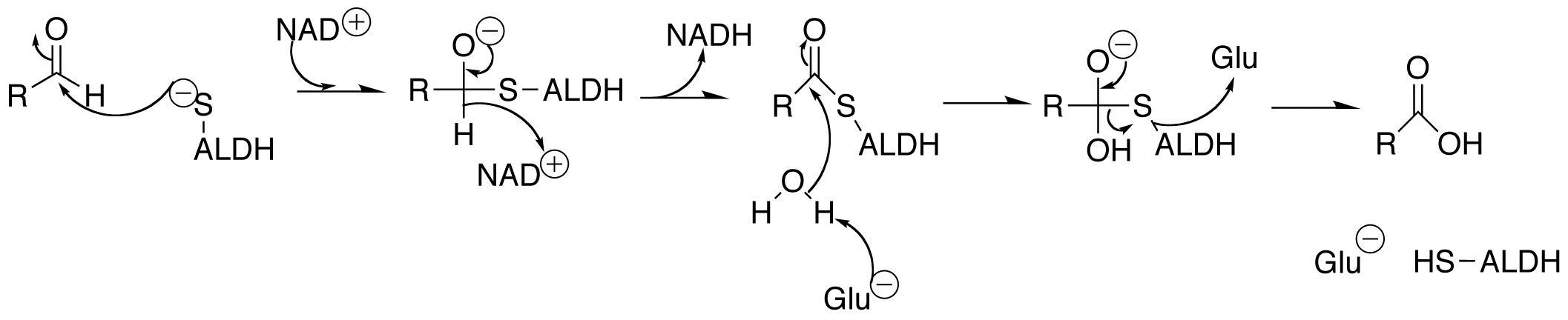 Aldehydes are the natural by-product of many physiological processes, as well as being the consequence of many industrial processes, put out into the environment in the form of smog and motor vehicle exhaust. Build-up of aldehydes in the brain and pericardium can be detrimental to a person's health, as they can form adducts with important molecules and cause their inactivation.
Considering how prevalent aldehydes are, there must be an enzyme to facilitate their oxidation to a less volatile compound.
Aldehydes are the natural by-product of many physiological processes, as well as being the consequence of many industrial processes, put out into the environment in the form of smog and motor vehicle exhaust. Build-up of aldehydes in the brain and pericardium can be detrimental to a person's health, as they can form adducts with important molecules and cause their inactivation.
Considering how prevalent aldehydes are, there must be an enzyme to facilitate their oxidation to a less volatile compound.
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
belonging to the group of oxidoreductases
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually uti ...
that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that ass ...
such as FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
catalyzes the oxidation of ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde.
IUBMB Classification
Oxidoreductases, enzymes that catalyze oxidation-reduction reactions, constitute Class EC 1 of the IUBMB classification of enzyme-catalyzed reactions. Any of these may be called dehydrogenases, especially those in which NAD+ is the electron acceptor (oxidant), but reductase is also used when the physiological emphasis on reduction of the substrate, andoxidase
In biochemistry, an oxidase is an enzyme that catalyzes oxidation-reduction reactions, especially one involving dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydro ...
is used ''only'' when O2 is the electron acceptor. The systematic name of an oxidoreductase is "donor:acceptor oxidoreductase", but when possible it is more conveniently named as "donor dehydrogenase".
Reactions catalyzed
Transferring a hydride and releasing a proton
Sometimes a dehydrogenase catalyzed reaction will look like this: AH + B+ ↔ A+ + BH when ahydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
is transferred.  A represents the substrate that will be oxidized, while B is the hydride acceptor. Note how when the hydride is transferred from A to B, the A has taken on a positive charge; this is because the enzyme has taken two electrons from the substrate in order to reduce the acceptor to BH.
The result of a dehydrogenase catalyzed reaction is not always the acquisition of a positive charge. Sometimes the substrate loses a proton. This may leave free electrons on the substrate that move into a double bond. This happens frequently when an alcohol is the substrate; when the proton on the oxygen leaves, the free electrons on the oxygen will be used to create a double bond, as seen in the oxidation of ethanol to acetaldehyde carried out by alcohol dehydrogenase in the image on the right.
Another possibility is that a water molecule will enter the reaction, contributing a hydroxide ion to the substrate and a proton to the environment. The net result on the substrate is the addition of one oxygen atom. This is seen for example in the oxidation of acetaldehyde to
A represents the substrate that will be oxidized, while B is the hydride acceptor. Note how when the hydride is transferred from A to B, the A has taken on a positive charge; this is because the enzyme has taken two electrons from the substrate in order to reduce the acceptor to BH.
The result of a dehydrogenase catalyzed reaction is not always the acquisition of a positive charge. Sometimes the substrate loses a proton. This may leave free electrons on the substrate that move into a double bond. This happens frequently when an alcohol is the substrate; when the proton on the oxygen leaves, the free electrons on the oxygen will be used to create a double bond, as seen in the oxidation of ethanol to acetaldehyde carried out by alcohol dehydrogenase in the image on the right.
Another possibility is that a water molecule will enter the reaction, contributing a hydroxide ion to the substrate and a proton to the environment. The net result on the substrate is the addition of one oxygen atom. This is seen for example in the oxidation of acetaldehyde to acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
by acetaldehyde dehydrogenase
Acetaldehyde dehydrogenases () are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetic acid. The oxidation of acetaldehyde to acetate can be summarized as follows:
Acetaldehyde + NAD+ + Coenzyme A ↔ Acetyl-CoA + NA ...
, a step in the metabolism of ethanol and in the production of vinegar.
Transferring two hydrogens
succinate dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates i ...
. The two hydrogens have been transferred to the carrier or the other product, with their electrons.
Identifying a dehydrogenase reaction
The distinction between the subclasses of oxidoreductases that catalyze oxidation reactions lies in their electron acceptors.oxidase
In biochemistry, an oxidase is an enzyme that catalyzes oxidation-reduction reactions, especially one involving dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydro ...
are easily distinguishable if one considers the electron acceptor. An oxidase will remove electrons from a substrate as well, but only uses oxygen as its electron acceptor. One such reaction is: AH2 + O2 ↔ A + H2O2.
Sometimes an oxidase reaction will look like this: 4A + 4H+ + O2 ↔ 4A+ + 2H2O. In this case, the enzyme is taking electrons from the substrate, and using free protons to reduce the oxygen, leaving the substrate with a positive charge. The product is water, instead of hydrogen peroxide as seen above. An example of an oxidase that functions like this is complex IV in the Electron Transport Chain (ETC
* Etc. or et cetera, a Latin expression meaning "and the other things" or "and the rest".
ETC or etc may also refer to:
Companies and organizations
* ETC (Chilean TV channel), a Chilean cable television channel
* ETC (Philippine TV channel), a P ...
).
Note that oxidases typically transfer the equivalent of dihydrogen (H2), and the acceptor is a dioxygen. Similarly, a peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides.
Functionality
Peroxidases typically ca ...
(another subclass of oxidoreductases) will use a peroxide (H2O2) as the electron acceptor, rather than an oxygen.
Electron acceptors
coenzymes
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that ass ...
that are often referred to as "redox cofactors."
NAD+
NAD+, or nicotinamide adenine dinucleotide, is a dinucleotide, containing two nucleotides. One of the nucleotides it contains is an adenine group, while the other is nicotinamide. In order to reduce this molecule, a hydrogen and two electrons must be added to the 6-carbon ring of nicotinamide; one electron is added to the carbon opposite the positively charged nitrogen, causing a rearrangement of bonds within the ring to give nitrogen more electrons; it will lose its positive charge as a result. The other electron is "stolen" from an additional hydrogen, leaving the hydrogen ion in solution.Reduction of NAD+: NAD+ + 2H+ + 2e− ↔ NADH + H+NAD+ is mostly used in catabolic pathways, such as
glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, that break down energy molecules to produce ATP. The ratio of NAD+ to NADH is kept very high in the cell, keeping it readily available to act as an oxidizing agent.
NADP+
NADP+ differs from NAD+ only in the addition of a phosphate group to the adenosine 5-membered carbon ring. The addition of the phosphate does not alter the electron transport abilities of the carrier. The phosphate group creates enough contrast between the two groups that they bind to the active site of different enzymes, generally catalyzing different types of reactions. These two electron carriers are easily distinguished by enzymes and participate in very different reactions. NADP+ mainly functions with enzymes that catalyze anabolic, or biosynthetic, pathways. Specifically, NADPH will act as a reducing agent in these reactions, resulting in NADP+. These are pathways that convert substrates to more complicated products, using ATP. The reasoning behind having two separate electron carriers for anabolic and catabolic pathways relates to regulation of metabolism. The ratio of NADP+ to NADPH in the cell is kept rather low, so that NADPH is readily available as a reducing agent; it is more commonly used as a reducing agent than NADP+ is used as an oxidizing agent.FAD
FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
, or flavin adenine dinucleotide, is a prosthetic group (a non-polypeptide unit bound to a protein that is required for function) that consists of an adenine nucleotide and a flavin mononucleotide. FAD is a unique electron acceptor. Its fully reduced form is FADH2 (known as the hydroquinone form), but FAD can also be partially oxidized as FADH by either reducing FAD or oxidizing FADH2. Dehydrogenases typically fully reduce FAD to FADH2. The production of FADH is rare.
The double-bonded nitrogen atoms in FAD make it a good acceptor in taking two hydrogen atoms from a substrate. Because it takes two atoms rather than one, FAD is often involved when a double bond is formed in the newly oxidized substrate. FAD is unique because it is reduced by two electrons ''and'' two protons, as opposed to both NAD+ and NADP, which only take one proton.
Examples
Biological implications
 Aldehydes are the natural by-product of many physiological processes, as well as being the consequence of many industrial processes, put out into the environment in the form of smog and motor vehicle exhaust. Build-up of aldehydes in the brain and pericardium can be detrimental to a person's health, as they can form adducts with important molecules and cause their inactivation.
Considering how prevalent aldehydes are, there must be an enzyme to facilitate their oxidation to a less volatile compound.
Aldehydes are the natural by-product of many physiological processes, as well as being the consequence of many industrial processes, put out into the environment in the form of smog and motor vehicle exhaust. Build-up of aldehydes in the brain and pericardium can be detrimental to a person's health, as they can form adducts with important molecules and cause their inactivation.
Considering how prevalent aldehydes are, there must be an enzyme to facilitate their oxidation to a less volatile compound. Aldehyde dehydrogenase
Aldehyde dehydrogenases () are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes (R–C(=O)) to carboxylic acids (R–C(=O)). The oxygen comes from a water molecule. To date, nineteen ALDH genes have b ...
s (ALDH) are NAD+ dependent enzymes that function to remove toxic aldehydes from the body, functioning mostly in the mitochondria of cells. These enzymes are largely responsible for the detoxification of acetylaldehyde, which is an intermediate in the metabolism of ethanol. It has been shown that a mutation in the ALDH2 gene (one of 19 aldehyde dehydrogenase genes) is what leads to the common occurrence in East Asian population of a flushed face after consuming alcohol, due to the build-up of acetaldehyde. This build-up of acetaldehyde also causes headaches and vomiting (hangover
A hangover is the experience of various unpleasant physiological and psychological effects usually following the consumption of alcohol, such as wine, beer, and liquor. Hangovers can last for several hours or for more than 24 hours. Typical sympto ...
symptoms) if not broken down quickly enough, another reason why those with acetaldehyde DH deficiencies have bad reactions to alcohol. Importantly, a lack of this enzyme has been linked to an increase in the risk of myocardial infarction
Infarction is tissue death (necrosis) due to inadequate blood supply to the affected area. It may be caused by artery blockages, rupture, mechanical compression, or vasoconstriction. The resulting lesion is referred to as an infarct
(from the ...
, while activation has shown the enzyme's ability to reduce damage caused by ischaemia
Ischemia American and British English spelling differences#ae and oe, or ischaemia is a restriction in blood supply to any tissue (biology), tissue, Skeletal muscle, muscle group, or Organ (biology), organ of the body, causing a shortage of oxyg ...
.
Deactivation of aldehyde dehydrogenases has been shown to be instrumental in the mechanisms of many cancers. ALDHs function in cell differentiation, proliferation, oxidation, and drug resistance. These enzymes are only one example of the many different types of dehydrogenases in the human body; their wide array of functions, and the impact that their deactivation or mutations has upon crucial cell processes underscores the importance of all dehydrogenases in maintaining body homeostasis.
More examples
*acetaldehyde dehydrogenase
Acetaldehyde dehydrogenases () are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetic acid. The oxidation of acetaldehyde to acetate can be summarized as follows:
Acetaldehyde + NAD+ + Coenzyme A ↔ Acetyl-CoA + NA ...
* alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
* Delta12-fatty acid dehydrogenase
* glutamate dehydrogenase
Glutamate dehydrogenase (GLDH, GDH) is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typical ...
(an enzyme that can convert glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
to α-Ketoglutarate Ketoglutaric acid or oxoglutaric acid, or its conjugate base, the carboxylate ketoglutarate or oxoglutarate, may refer to the following chemical compounds:
* α-Ketoglutaric acid, an intermediate in the citric acid cycle
The citric acid cycle (CA ...
and vice versa).
* lactate dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from on ...
(used to convert NADH back to NAD+ in anaerobic glycolysis, and in the back reaction to produce NADH)
* pyruvate dehydrogenase
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.
Pyruvate dehydrogenase is us ...
(A common enzyme that feeds the TCA Cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
by converting pyruvate to acetyl CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
, using NAD+. In this reaction, the substrate not only is oxidized but also loses a carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
molecule, and is attached to the CoA coenzyme.)
* glucose-6-phosphate dehydrogenase
Glucose-6-phosphate dehydrogenase (G6PD or G6PDH) () is a cytosolic enzyme that catalyzes the chemical reaction
: D-glucose 6-phosphate + NADP+ + H2O 6-phospho-D-glucono-1,5-lactone + NADPH + H+
This enzyme participates in the pentose phospha ...
(involved in the pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
, producing NADPH)
* glyceraldehyde-3-phosphate dehydrogenase
Glyceraldehyde 3-phosphate dehydrogenase (abbreviated GAPDH) () is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long establish ...
(involved in glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, uses NAD+)
* sorbitol dehydrogenase
Sorbitol dehydrogenase (or SDH) is a cytosolic enzyme. In humans this protein is encoded by the ''SORD'' gene.
Sorbitol dehydrogenase is an enzyme in carbohydrate metabolism converting sorbitol, the sugar alcohol form of glucose, into fructose ...
TCA cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
examples:
* isocitrate dehydrogenase
Isocitrate dehydrogenase (IDH) () and () is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a s ...
(uses NAD+, also has an isozyme In biochemistry, isozymes (also known as isoenzymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters (e.g. dif ...
that uses NADP)
* alpha-ketoglutarate dehydrogenase
The oxoglutarate dehydrogenase complex (OGDC) or α-ketoglutarate dehydrogenase complex is an enzyme complex, most commonly known for its role in the citric acid cycle.
Units
Much like pyruvate dehydrogenase complex (PDC), this enzyme forms a co ...
(uses NAD+)
* succinate dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates i ...
(uses FAD)
* malate dehydrogenase
Malate dehydrogenase () (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate ...
(uses NAD+)
References