|
Committed Effective Dose Equivalent (CEDE)
Committed dose equivalent and Committed effective dose equivalent are dose quantities used in the United States system of radiological protection for irradiation due to an internal source. Committed dose equivalent (CDE) CDE is defined by the United States Nuclear Regulatory Commission in Title 10, Section 20.1003, of the Code of Federal Regulations (10 CFR 20.1003), such that "The Committed dose equivalent, CDE (HT,50) is the dose to some specific organ or tissue of reference (T) that will be received from an intake of radioactive material by an individual during the 50-year period following the intake". "The calculation of the committed effective dose equivalent (CEDE) begins with the determination of the equivalent dose, HT, to a tissue or organ, T. Where DT,R is the absorbed dose in rads (one gray, an SI unit, equals 100 rads) averaged over the tissue or organ, T, due to radiation type, R, and WR is the radiation weighting factor. The unit of equivalent dose is the rem ( siever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
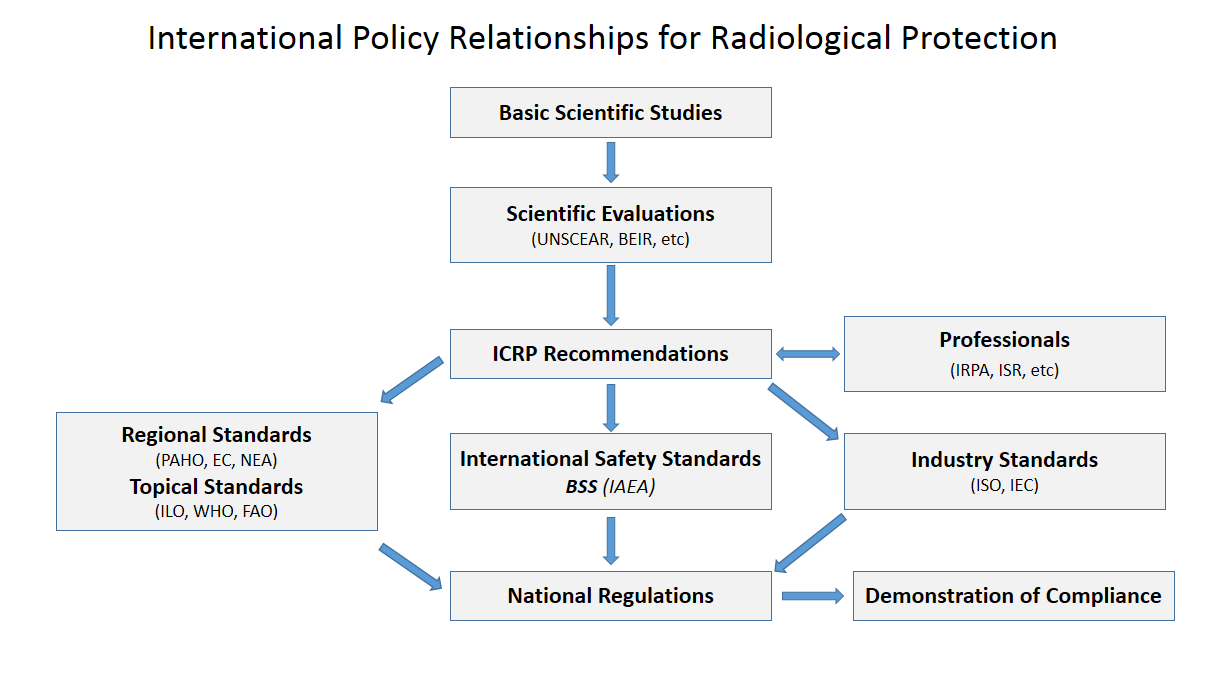
Radiological Protection
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination. Ionizing radiation is widely used in industry and medicine, and can present a significant health hazard by causing microscopic damage to living tissue. There are two main categories of ionizing radiation health effects. At high exposures, it can cause "tissue" effects, also called "deterministic" effects due to the certainty of them happening, conventionally indicated by the unit gray and resulting in acute radiation syndrome. For low level exposures there can be statistically elevated risks of radiation-induced cancer, called "stochastic effects" due to the uncertainty of them happening, conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionizing Radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy ultraviolet part of the electromagnetic spectrum are ionizing radiation, whereas the lower energy ultraviolet, visible light, nearly all types of laser light, infrared, microwaves, and radio waves are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area is not sharply defined, as different molecules and atoms ionize at different energies. The energy of ionizing radiation starts between 10 electronvolts (eV) and 33 eV. Typical ionizing subatomic particles include alpha particles, beta particles, and neutrons. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thoron
There are 37 known isotopes of radon (86Rn), from 195Rn to 231Rn; all are radioactive. The most stable isotope is 222Rn with a half-life of 3.823 days, which decays into . Five isotopes of radon, 217, 218, 219, 220, 222Rn occur in trace quantities in nature as decay products of, respectively, 217At, 218At, 223Ra, 224Ra, and 226Ra. 217Rn is produced in a rare branch in the decay chain of trace quantities of 237Np; 218Rn and 222Rn are intermediate steps in the decay chain for 238U; 219Rn is an intermediate step in the decay chain for 235U; and 220Rn occurs in the decay chain for 232Th. List of isotopes , - , 195Rn , , style="text-align:right" , 86 , style="text-align:right" , 109 , 195.00544(5) , 6 ms , , , 3/2−# , , - , style="text-indent:1em" , 195mRn , , colspan="3" style="text-indent:2em" , 50(50) keV , 6 ms , , , 13/2+# , , - , rowspan=2, 196Rn , rowspan=2, , rowspan=2 style="text-align:right" , 86 , rowspan=2 style="text-alig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) They are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided. All known thorium isotopes are unstable. The most stable isotope, 232Th, has a half-life of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium are the only significantly radioactive elements that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sands as a by-product of extracti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. In nature, uranium is found as uranium-238 (99. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous. Plutonium was first synthetically produced and isolated in late 1940 and early 1941, by a deuteron bombardment of uranium-238 in the cyclotron at the University of California, Berkeley. First, neptunium-238 ( half-life 2.1 days) was synthesized, which subsequently beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Explosive
A nuclear explosive is an explosive device that derives its energy from nuclear reactions. Almost all nuclear explosive devices that have been designed and produced are nuclear weapons intended for warfare. Other, non-warfare, applications for nuclear explosives have occasionally been proposed. For example, nuclear pulse propulsion is a form of spacecraft propulsion that would use nuclear explosives to provide impulse to a spacecraft. A similar application is the proposal to use nuclear explosives for asteroid deflection. From 1958 to 1965 the United States government ran a project to design a nuclear explosive powered nuclear pulse rocket called Project Orion. Never built, this vessel would use repeated nuclear explosions to propel itself and was considered surprisingly practical. It is thought to be a feasible design for interstellar travel. Nuclear explosives were once considered for use in large-scale excavation. A nuclear explosion could be used to create a harbor, or a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Power Plant
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a electric generator, generator that produces electricity. , the International Atomic Energy Agency reported there were 422 nuclear power reactors in operation in 32 countries around the world, and 57 nuclear power reactors under construction. Nuclear plants are very often used for base load since their operations, maintenance, and fuel costs are at the lower end of the spectrum of costs. However, building a nuclear power plant often spans five to ten years, which can accrue to significant financial costs, depending on how the initial investments are financed. Nuclear power plants have a carbon footprint comparable to that of renewable energy such as photovoltaic power station, solar farms and wind farms, and much lower than fossil fuels such as gas-fired ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |