|
Alpha-Ethylphenethylamine
Phenylisobutylamine, also known as α-ethylphenethylamine, Butanphenamine, B or AEPEA, is a stimulant drug of the phenethylamine class. It is a higher homologue of amphetamine, differing from amphetamine's molecular structure only by the substitution of the methyl group at the alpha position of the side chain with an ethyl group. Compared to amphetamine, phenylisobutylamine has strongly reduced dopaminergic effects, and instead acts as a selective norepinephrine releasing agent. The dextroisomer of phenylisobutylamine partially substitutes for dextroamphetamine in rats. A number of derivatives of phenylisobutylamine are known, including BDB, MBDB, EBDB, butylone (βk-MBDB), eutylone (βk-EBDB), Ariadne (α-Et-DOM), 4-CAB, and 4-MAB. "Phenylisobutylamine" is in fact a chemical misnomer because isobutylamine itself contains a branched chain. The correct name after this style for this class of compound would be "phenyl''sec''butylamine". See also * Phenethylamine * Amphet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzodioxolylbutanamine
1,3-Benzodioxolylbutanamine (also known as 3,4-methylenedioxybutanphenamine, MDB, BDB, J, and 3,4-methylenedioxy-α-ethylphenethylamine) is an entactogenic drug of the phenethylamine chemical class. It is the α-ethyl analog of MDPEA and MDA and the methylenedioxy analogue of α-ethylphenethylamine. BDB was first synthesized by Alexander Shulgin. In his book '' PiHKAL'', the dosage range is listed as 150–230 mg and the duration is listed as 4–8 hours. BDB produces entactogenic, MDMA-like effects. Although pleasant and euphoric, BDB is also fairly sedating and some users feel that the lack of stimulant effect makes it less enjoyable than other similar drugs. Additional side effects associated with BDB include nystagmus and dizziness. Very little data exists about the pharmacological properties, metabolism, and toxicity of BDB. Animal studies and anecdotal reports show that BDB is a slightly more potent serotonin releasing agent than its methylated sister compound m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mouth
In animal anatomy, the mouth, also known as the oral cavity, or in Latin cavum oris, is the opening through which many animals take in food and issue vocal sounds. It is also the cavity lying at the upper end of the alimentary canal, bounded on the outside by the lips and inside by the pharynx. In tetrapods, it contains the tongue and, except for some like birds, teeth. This cavity is also known as the buccal cavity, from the Latin ''bucca'' ("cheek"). Some animal phyla, including arthropods, molluscs and chordates, have a complete digestive system, with a mouth at one end and an anus at the other. Which end forms first in ontogeny is a criterion used to classify bilaterian animals into protostomes and deuterostomes. Development In the first multicellular animals, there was probably no mouth or gut and food particles were engulfed by the cells on the exterior surface by a process known as endocytosis. The particles became enclosed in vacuoles into which enzymes were secr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextroamphetamine
Dextroamphetamine is a central nervous system (CNS) stimulant and an amphetamine enantiomer that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used as an athletic performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. The amphetamine molecule exists as two enantiomers, levoamphetamine and dextroamphetamine. Dextroamphetamine is the dextrorotatory, or 'right-handed', enantiomer and exhibits more pronounced effects on the central nervous system than levoamphetamine. Pharmaceutical dextroamphetamine sulfate is available as both a brand name and generic drug in a variety of dosage forms. Dextroamphetamine is sometimes prescribed as the inactive prodrug lisdexamfetamine dimesylate, which is converted into dextroamphetamine after absorption. Dextroamphetamine, like other amphetamines, elicits its stimulating effects via several distinct actions: it inhibits or reverses the transport ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphetamine
Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used as a recreational drug. Amphetamine was discovered in 1887 and exists as two enantiomers: levoamphetamine and dextroamphetamine. ''Amphetamine'' properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation. Phenethylamine is sold as a dietary supplement for purported mood and weight loss-related therapeutic benefits; however, in orally ingested phenethylamine, a significant amount is metabolized in the small intestine by monoami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutylamine
Isobutylamine is an organic chemical compound (specifically, an amine) with the formula (CH3)2CHCH2NH2, and occurs as a colorless liquid. Chemblink.com Isobutylamine is one of the four isomeric amines of , the others being ''n''-butylamine, ''sec''-butylamine and ''tert''-butylamine. It is the decarboxylated form of the |
4-methylphenylisobutylamine
4-Methylphenylisobutylamine (4-MAB), also known as 4-methyl-α-ethylphenethylamine, is a stimulant drug of the phenethylamine class. See also * Phenylisobutylamine * 4-Methylamphetamine * Benzodioxolylbutanamine 1,3-Benzodioxolylbutanamine (also known as 3,4-methylenedioxybutanphenamine, MDB, BDB, J, and 3,4-methylenedioxy-α-ethylphenethylamine) is an entactogenic drug of the phenethylamine chemical class. It is the α- ethyl analog of MDPEA and MD ... References {{DEFAULTSORT:Methylphenylisobutylamine, 4- Substituted amphetamines Entactogens and empathogens Serotonin-norepinephrine-dopamine releasing agents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-chlorophenylisobutylamine
4-Chlorophenylisobutylamine (4-CAB, AEPCA), also known as 4-chloro-α-ethylphenethylamine, is an entactogen and stimulant drug of the phenethylamine class. It is an analogue of ''para''-chloroamphetamine (PCA) where the alpha position methyl has been replaced with an ethyl group. In comparison to PCA, 4-CAB is approximately 2- and 5-fold less potent at inhibiting the reuptake of serotonin ( IC50 = 330 nM) and dopamine (IC50 = 2,343 nM), respectively, and is about 3-fold less potent in substituting for MDMA in animals in drug discrimination assays. Though its dopaminergic activity is significantly attenuated compared to PCA, unlike the case of MBDB, it is not abolished, and is actually similar to that of MDMA. Relative to PCA, 4-CAB is also substantially less effective as a serotonergic neurotoxin. A single 10 mg/kg administration of PCA to rats produces an approximate 80% decrease in serotonin markers as observed 1 week later. In contrast, 11 mg/kg and 22 mg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ariadne (psychedelic)
Ariadne (also known as 4C-D, 4C-DOM, α-Et-2C-D, BL-3912, or dimoxamine) is a lesser-known psychedelic drug. It is a homologue of 2C-D and DOM. Ariadne was first synthesized by Alexander Shulgin. In his book '' PiHKAL'', Shulgin reported testing Ariadne up to a dose of 32 mg, and reported that it produces psychedelia at a bare threshold. Very little data exists about the pharmacological properties, metabolism, and toxicity of Ariadne in humans apart from Shulgin's limited testing. Shulgin reported that the drug was tested by Bristol Laboratories as an antidepressant, in an anecdote where he was explaining how human testing is invaluable (compared to animal testing) on drugs that change the state of the mind. He said, "Before they launched into a full multi-clinic study to determine if it's going to be worth the animal studies or not, every person on the board of directors took it." In an animal study, Ariadne was shown to produce stimulus generalization in rats trained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eutylone
Eutylone (also known as β-keto-1,3-benzodioxolyl-''N''-ethylbutanamine, bk-EBDB, and ''N''-ethylbutylone) is a stimulant and empathogenic compound developed in the 1960s, which is classified as a designer drug. It was first reported to the EMCDDA in 2014 and became widespread internationally in 2019-2020 following bans on the related compound ephylone. It is not a natural, but a synthetic cathinone. In 2021, eutylone was the most common cathinone identified by the Drug Enforcement Administration in the United States. Legal status Sweden's public health agency suggested classifying eutylone as a hazardous substance, on September 25, 2019. In the United States Eutylone is considered a schedule 1 controlled substance as a positional isomer of Pentylonehttps://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf See also * 5-Methylethylone * Butylone * Ethyl-J * Ethylone * Ephylone ''N''-Ethylpentylone (β-keto-ethylbenzodioxolylpentanamine, βk-ethyl-K, βk-E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
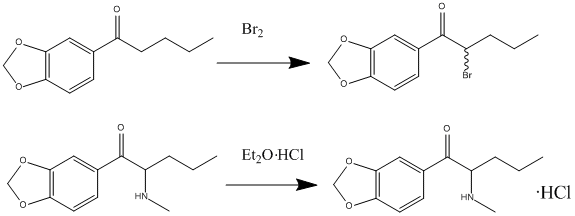
Butylone
Butylone, also known as β-keto-''N''-methylbenzodioxolylbutanamine (βk-MBDB), is an entactogen, psychedelic, and stimulant psychoactive drug of the phenethylamine chemical class. It is the β-keto (substituted cathinone) analogue of MBDB and the substituted methylenedioxyphenethylamine analogue of buphedrone. History Butylone was first synthesized by Koeppe, Ludwig and Zeile which is mentioned in their 1967 paper. It remained an obscure product of academia until 2005 when it was sold as a designer drug. Butylone shares the same relationship to MBDB as methylone does to MDMA ("Ecstasy"). Formal research on this chemical was first conducted in 2009, when it was shown to be metabolised in a similar manner to related drugs like methylone. Synthesis Butylone can be synthesized in a laboratory via the following route: 3,4-methylenedioxybutyrophenone dissolved in dichloromethane to bromine gives 3′,4′-methylenedioxy-2-bromobutyrophenone. This product was then dissolved in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylbenzodioxolylbutanamine
Ethylbenzodioxolylbutanamine (EBDB; Ethyl-J) is a lesser-known entactogen, stimulant, and psychedelic. It is the ''N''-ethyl analogue of benzodioxylbutanamine (BDB; "J"), and also the α-ethyl analogue of methylenedioxyethylamphetamine (MDEA; "Eve"). EBDB was first synthesized by Alexander Shulgin. In his book '' PiHKAL'', the minimum dosage consumed was 90 mg, and the duration is unknown. EBDB produced few to no effects at the dosage range tested in ''PiHKAL'', but at higher doses of several hundred milligrams it produces euphoric effects similar to those of methylbenzodioxylbutanamine (MBDB; "Eden", "Methyl-J"), although milder and shorter lasting. Very little data exists about the pharmacological properties, metabolism, and toxicity of EBDB. See also * Methylbenzodioxolylbutanamine (MBDB; Methyl-J) * Ethylbenzodioxolylpentanamine (EBDP; Ethyl-K) * Eutylone Eutylone (also known as β-keto-1,3-benzodioxolyl-''N''-ethylbutanamine, bk-EBDB, and ''N''-ethylbutylone) is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |