|
Zn-65
Naturally occurring zinc (30Zn) is composed of the 5 stable isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn with 64Zn being the most abundant (48.6% natural abundance). Twenty-five radioisotopes have been characterised with the most abundant and stable being 65Zn with a half-life of 244.26 days, and 72Zn with a half-life of 46.5 hours. All of the remaining radioactive isotopes have half-lives that are less than 14 hours and the majority of these have half-lives that are less than 1 second. This element also has 10 meta states. Zinc has been proposed as a "Salted bomb, salting" material for nuclear weapons. A jacket of Isotope separation, isotopically enriched 64Zn, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 65Zn with a half-life of 244 days and produce approximately 1.115 MeV of Gamma ray, gamma radiation, significantly increasing the radioactivity of the weapon's Nuclear fallout, fallout f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
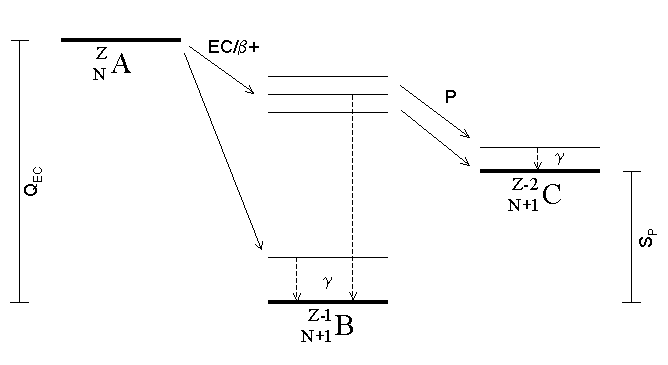
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous and induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei which can decay by this process are described as lying beyond the neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Observationally Stable
Stable nuclides are nuclides that are not radioactive and so (unlike radionuclides) do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been known to decay using current equipment (see list at the end of this article). Of these 80 elements, 26 have only one stable isotope; they are thus termed monoisotopic. The rest have more than one stable isotope. Tin has ten stable isotopes, the largest number of stable isotopes known for an element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 34 more (total of 286) are known to be radioactive with sufficiently long half-lives (also known) to occur primordially. If the half-life of a nuclide is comparable to, or greater ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon-burning Process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the ''main sequence'' on the Hertzsprung–Russell diagram. It follows the previous stages of hydrogen, helium, carbon, neon and oxygen burning processes. Silicon burning begins when gravitational contraction raises the star's core temperature to 2.7–3.5 billion kelvin ( GK). The exact temperature depends on mass. When a star has completed the silicon-burning phase, no further fusion is possible. The star catastrophically collapses and may explode in what is known as a Type II supernova. Nuclear fusion sequence and silicon photodisintegration After a star completes the oxygen-burning process, its core is composed primarily of silicon and sulfur. If it has sufficiently high mass, it further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Assumption University Journal Of Technology
Assumption, in Christianity, refers to the Assumption of Mary, a belief in the taking up of the Virgin Mary into heaven. Assumption may also refer to: Places * Assumption, Alberta, Canada * Assumption, Illinois, United States ** Assumption Township, Christian County, Illinois * Assumption Island, Seychelles ** Assumption Island Airport * Assumption, Minnesota, United States * Assumption, Nebraska, United States * Assumption, Ohio, United States * Assumption Parish, Louisiana, United States Arts, entertainment, and media * "Assumption" (short story), a 1929 story by Samuel Beckett * Assumption of Moses, a Jewish apocryphal pseudepigraphical work of uncertain date and authorship Churches * Assumption Chapel, Minnesota, United States * Assumption of the Blessed Virgin Mary Church, Michigan, United States * Assumption – St. Paul, New York, United States * Cathedral of the Assumption (other) * Church of the Assumption (other) Logic * Closed-world assumptio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fallout
Nuclear fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and the shock wave has passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes. The amount and spread of fallout is a product of the size of the weapon and the altitude at which it is detonated. Fallout may get entrained with the products of a pyrocumulus cloud and fall as black rain (rain darkened by soot and other particulates, which fell within 30–40 minutes of the atomic bombings of Hiroshima and Nagasaki). This radioactive dust, usually consisting of fission products mixed with bystanding atoms that are neutron-activated by exposure, is a form of radioactive contamination. Types of fallout Fallout comes in two varieties. The first is a small amount of carcinogenic material with a long half-life. The second, depending on the height of detonation, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermonuclear Weapon
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lower mass, or a combination of these benefits. Characteristics of nuclear fusion reactions make possible the use of non-fissile depleted uranium as the weapon's main fuel, thus allowing more efficient use of scarce fissile material such as uranium-235 () or plutonium-239 (). The first full-scale thermonuclear test was carried out by the United States in 1952; the concept has since been employed by most of the world's nuclear powers in the design of their weapons. Modern fusion weapons consist essentially of two main components: a nuclear fission primary stage (fueled by or ) and a separate nuclear fusion secondary stage containing thermonuclear fuel: the heavy hydrogen isotopes deuterium and tritium, or in modern weapons lithium deuteride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |