|
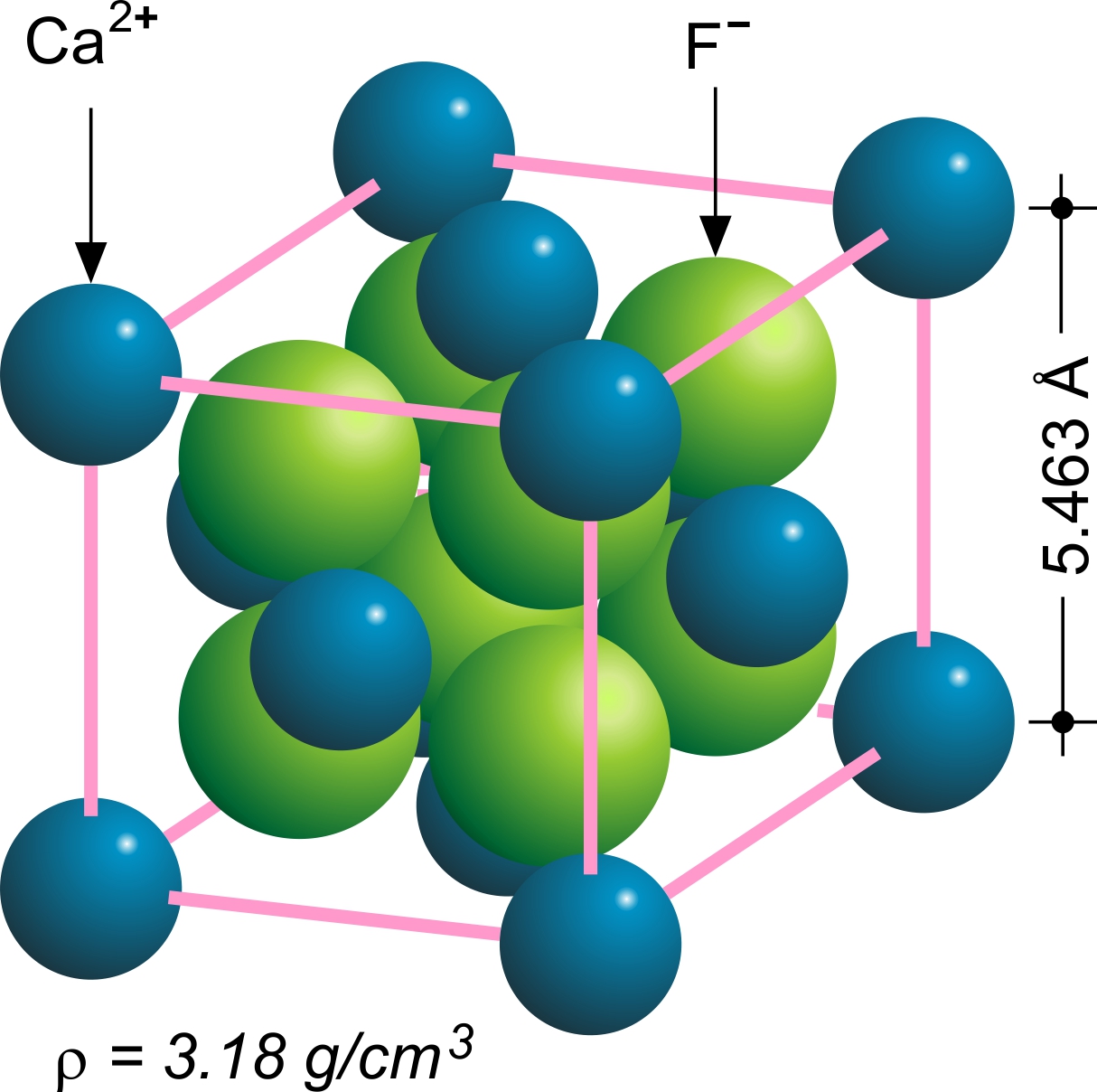
Yttrium(III) Fluoride
Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula Y F3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY(F,Cl)6. Sometimes mineral fluorite contains admixtures of yttrium. Synthesis YF3 can be produced by reacting fluorine with yttria or yttrium hydroxide with hydrofluoric acid. :Y(OH)3 + 3HF → YF3 + 3H2O Occurrence and uses It occurs as the mineral waimirite-(Y). Yttrium(III) fluoride can be used for the production of metallic yttrium, thin films, glasses and ceramics. Hazards Conditions/substances to avoid are: acids, active metals and moisture Moisture is the presence of a liquid, especially water, often in trace amounts. Small amounts of water may be found, for example, in the air (humidity), in foods, and in some commercial products. Moisture also refers to the amount of water vapo .... References Fluorides Metal hal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nomenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moisture
Moisture is the presence of a liquid, especially water, often in trace amounts. Small amounts of water may be found, for example, in the air (humidity), in foods, and in some commercial products. Moisture also refers to the amount of water vapor present in the air. Moisture control in products Control of moisture in products can be a vital part of the process of the product. There is a substantial amount of moisture in what seems to be dry matter. Ranging in products from cornflake cereals to washing powders, moisture can play an important role in the final quality of the product. There are two main aspects of concern in moisture control in products: allowing too much moisture or too little of it. For example, adding some water to cornflake cereal, which is sold by weight, reduces costs and prevents it from tasting too dry, but adding too much water can affect the crunchiness of the cereal and the freshness because water content contributes to bacteria growth. Water content o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acids
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material polytetrafluoroethylene, PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to Etching (microfabrication), etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Polytetrafluoroethylene, Teflon, fluoropolymers, fluorocarbons, and refrigeration, refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium Hydroxide
Yttrium(III) hydroxide is an inorganic compound and an alkali with the chemical formula Y(OH)3. Production Yttrium(III) hydroxide can be produced by reacting yttrium(III) nitrate and sodium hydroxide in aqueous solution:田俐,陈稳纯,陈琳 等. 水热法合成氢氧化钇纳米管. 无机材料学报. 2009. 24(2): 335-339 : Y(NO3)3 + 3 NaOH → Y(OH)3↓ + 3 NaNO3 This gives yttrium(III) hydroxide as a white gelatinous precipitate, which can be dried to a white powder. Chemical properties Yttrium(III) hydroxide is an alkali, so it can react with acid: : Y(OH)3 + 3 HNO3 → Y(NO3)3 + 3 H2O : 2 Y(OH)3 + 3 H2SO4 → Y2(SO4)3 + 3 H2O The compound absorbs atmospheric carbon dioxide Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar .... When heated, yttrium(III) hydroxide will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttria
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Uses Phosphors Yttria is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red color in color TV picture tubes. Yttria lasers Y2O3 is a prospective solid-state laser material. In particular, lasers with ytterbium as dopant allow the efficient operation both in continuous operation and in pulsed regimes. At high concentration of excitations (of order of 1%) and poor cooling, the quenching of emission at laser frequency and avalanche broadband emission takes place. (Yttria-based lasers are not to be confused with YAG lasers using yttrium aluminum garnet, a widely used crystal host for rare earth laser dopants). Gas Lighting The original use of the mineral yttria and the purpose of its extraction from mineral sources was as part of the process of making gas mantles and other products for turning the flames of artifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals, and is never found in nature as a free element. 89Y is the only stable isotope, and the only isotope found in the Earth's crust. The most important uses of yttrium are LEDs and phosphors, particularly the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and tracing various materials to enhance their properties. Yttrium has no known biological role. Exposure to yttrium compounds can cause lung disease in humans. The element is named after '' ytterbite'', a mineral first identified in 1787 by the chemist Carl Axel Arrhenius. He n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_0468.jpg)