|
Yttria
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Uses Phosphors Yttria is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red color in color TV picture tubes. Yttria lasers Y2O3 is a prospective solid-state laser material. In particular, lasers with ytterbium as dopant allow the efficient operation both in continuous operation and in pulsed regimes. At high concentration of excitations (of order of 1%) and poor cooling, the quenching of emission at laser frequency and avalanche broadband emission takes place. (Yttria-based lasers are not to be confused with YAG lasers using yttrium aluminum garnet, a widely used crystal host for rare earth laser dopants). Gas Lighting The original use of the mineral yttria and the purpose of its extraction from mineral sources was as part of the process of making gas mantles and other products for turning the flames of artifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttria-stabilized Zirconia
Yttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" ( Zr O2) and "yttria" ( Y2 O3), hence the name. Stabilization Pure zirconium dioxide undergoes a phase transformation from monoclinic (stable at room temperature) to tetragonal (at about 1173 °C) and then to cubic (at about 2370 °C), according to the scheme : monoclinic (1173 °C) ↔ tetragonal (2370 °C) ↔ cubic (2690 °C) ↔ melt. Obtaining stable sintered zirconia ceramic products is difficult because of the large volume change, about 5%, accompanying the transition from tetragonal to monoclinic. Stabilization of the cubic polymorph of zirconia over wider range of temperatures is accomplished by substitution of some of the Zr4+ ions (ionic radius of 0.82 Å, too small for ideal lattice of fluorite characteristic for the cubi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals, and is never found in nature as a free element. 89Y is the only stable isotope, and the only isotope found in the Earth's crust. The most important uses of yttrium are LEDs and phosphors, particularly the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and tracing various materials to enhance their properties. Yttrium has no known biological role. Exposure to yttrium compounds can cause lung disease in humans. The element is named after '' ytterbite'', a mineral first identified in 1787 by the chemist Carl Axel Arrhenius. He n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
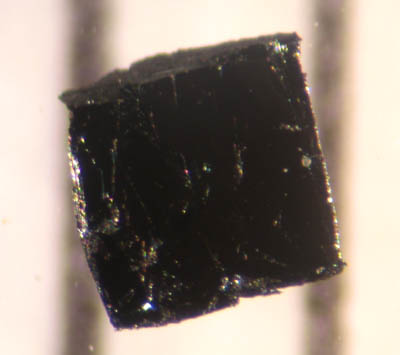
Yttriaite-(Y)
Yttriaite-(Y) is an exceedingly rare mineral, a natural form of yttrium oxide, Y2O3.Mills, S.J., Kartashov, P.M., Ma, C., Rossman, G.R., Novgorodova, M.I., Kampf, A.R., and Raudsepp, M., 2011: Yttriaite-(Y): The natural occurrence of Y2O3 from the Bol’shaya Pol’ya River, Subpolar Urals, Russia. American Mineralogist 96(7), 1166-1170 In terms of chemistry it is yttrium-analogue of kangite, arsenolite, avicennite and senarmontite (isometric minerals). Other minerals with the general formula A2O3 include corundum, bismite, bixbyite, eskolaite, hematite, karelianite, sphaerobismoite, tistarite, and valentinite Valentinite is an antimony oxide mineral with formula Sb2 O3. Valentinite crystallizes in the orthorhombic system and typically forms as radiating clusters of euhedral crystals or as fibrous masses. It is colorless to white with occasional shades ....Mindat, Tistarite, http://www.mindat.org/min-38695.html Yttriaite-(Y) forms tiny inclusions in native tungsten. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium Oxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant. Production, chemical properties, occurrence Zirconia is produced by calcining zirconium compounds, exploiting its high thermostability.Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Structure Three phases are known: monoclinic below 1170 °C, tetragonal between 1170 °C and 2370 °C, and cubic above 2370 °C. The trend is for higher symmetry at higher temperatures, as is usually the case. A small percentage of the oxides of calcium or yttrium stabilize in the cubic phase. The very rare mineral tazheranite, , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Continuous Wave
A continuous wave or continuous waveform (CW) is an electromagnetic wave of constant amplitude and frequency, typically a sine wave, that for mathematical analysis is considered to be of infinite duration. It may refer to e.g. a laser or particle accelerator having a continuous output, as opposed to a pulsed output. Continuous wave is also the name given to an early method of radio transmission, in which a sinusoidal carrier wave is switched on and off. Information is carried in the varying duration of the on and off periods of the signal, for example by Morse code in early radio. In early wireless telegraphy radio transmission, CW waves were also known as "undamped waves", to distinguish this method from damped wave signals produced by earlier ''spark gap'' type transmitters. Radio Transmissions before CW Very early radio transmitters used a spark gap to produce radio-frequency oscillations in the transmitting antenna. The signals produced by these spark-gap transmitters c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placer Deposit
In geology, a placer deposit or placer is an accumulation of valuable minerals formed by gravity separation from a specific source rock during sedimentary processes. The name is from the Spanish word ''placer'', meaning "alluvial sand". Placer mining is an important source of gold, and was the main technique used in the early years of many gold rushes, including the California Gold Rush. Types of placer deposits include alluvium, eluvium, beach placers, aeolian placers and paleo-placers. Placer materials must be both dense and resistant to weathering processes. To accumulate in placers, mineral particles must have a specific gravity above 2.58. Placer environments typically contain black sand, a conspicuous shiny black mixture of iron oxides, mostly magnetite with variable amounts of ilmenite and hematite. Valuable mineral components often occurring with black sands are monazite, rutile, zircon, chromite, wolframite, and cassiterite. Early mining operations were likely a result ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium(III) Chloride
Yttrium(III) chloride is an inorganic compound of yttrium and chloride. It exists in two forms, the hydrate (YCl3(H2O)6) and an anhydrous form (YCl3). Both are colourless solids that are highly soluble in water and deliquescent. Structure Solid YCl3 adopts a cubic structure with close-packed chloride ions and yttrium ions filling one third of the octahedral holes and the resulting YCl6 octahedra sharing three edges with adjacent octahedra, giving it a layered structure. This structure is shared by a range of compounds, notably aluminium trichloride, AlCl3. Preparation and reactions YCl3 is often prepared by the "ammonium chloride route," starting from either Y2O3 or hydrated chloride or oxychloride. or YCl3·6H2O. These methods produce (NH4)2[YCl5]: :10 NH4Cl + Y2O3 → 2 (NH4)2[YCl5] + 6 NH3 + 3 H2O :YCl3·6H2O + ''2'' NH4Cl → (NH4)2[YCl5] + 6 H2O The pentachloride thermal decomposition, decomposes thermally according to the following equation: : (NH4)2[YCl5] → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Temperature Superconductor
High-temperature superconductors (abbreviated high-c or HTS) are defined as materials that behave as superconductors at temperatures above , the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previously known superconductors, which function at even colder temperatures close to absolute zero. In absolute terms, these "high temperatures" are still far below ambient, and therefore require cooling. The first high-temperature superconductor was discovered in 1986, by IBM researchers Johannes Georg Bednorz, Bednorz and Karl Alexander Müller, Müller, who were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-c materials are type-II superconductors. The major advantage of high-temperature superconductors is that they can be cooled by using liquid nitrogen, as opposed to the previously known superconductors which require expensive and hard-to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_diagram.jpg)