|
Xanthine
Xanthine ( or , from Ancient Greek for its yellowish-white appearance; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine. Xanthine is a product on the pathway of purine degradation. * It is created from guanine by guanine deaminase. * It is created from hypoxanthine by xanthine oxidoreductase. * It is also created from xanthosine by purine nucleoside phosphorylase. Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme. Use and production Xanthine is used as a drug precursor for human and animal medications, and is produced as a pesticide ingredient. Clinical significance Derivatives of xanthine (known collectively as xanthines) are a group of alkaloids commonly used for their effects as mild stimulants and as bronchodila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraxanthine
Paraxanthine, also known as 1,7-dimethylxanthine, is an isomer of theophylline and theobromine, two well-known stimulants found in coffee, tea, and chocolate, mainly in the form of caffeine. It is a member of the xanthine family of alkaloids, which also includes theophylline and theobromine in addition to caffeine. Production and metabolism Paraxanthine is not known to be produced by plants but is observed in nature as a metabolite of caffeine in animals and some species of bacteria. Paraxanthine is the primary metabolite of caffeine in humans and other animals, such as mice. Shortly after ingestion, roughly 84% of caffeine is metabolized into paraxanthine by hepatic cytochrome P450, which removes a methyl group from the N3 position of caffeine. After formation, paraxanthine can be broken down to 7-methylxanthine by demethylation of the N1 position, which is subsequently demethylated into xanthine or oxidized by CYP2A6 and CYP1A2 into 1,7-dimethyluric acid. In another pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine Oxidoreductase
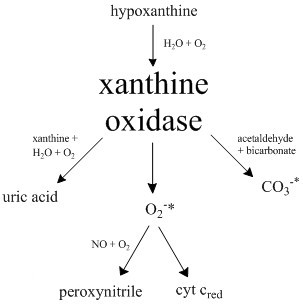
Xanthine oxidase (XO or XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. "XDH xanthine dehydrogenase" Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 xanthine + H2O2 * xanthine + H2O + O2 uric acid + H2O2 * Xanthine oxidase can a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine Oxidase
Xanthine oxidase (XO or XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. "XDH xanthine dehydrogenase" Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 xanthine + H2O2 * xanthine + H2O + O2 uric acid + H2O2 * Xanthine oxidase ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine chemical classification, class and is the most commonly consumed Psychoactive drug, psychoactive substance globally. It is mainly used for its eugeroic (wakefulness promoting), ergogenic (physical performance-enhancing), or nootropic (cognitive-enhancing) properties. Caffeine acts by blocking the binding of adenosine at a number of adenosine receptor types, inhibiting the centrally depressant effects of adenosine and enhancing the release of acetylcholine. Caffeine has a three-dimensional structure similar to that of adenosine, which allows it to bind and block its receptors. Caffeine also increases Cyclic adenosine monophosphate, cyclic AMP levels through nonselective Phosphodiesterase inhibitor, inhibition of phosphodiesterase, increases calcium release from intracellular stores, and Receptor antagonist, antagonizes GABA receptor, GABA receptors, although these mechanisms typically occur at concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids, where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-hydroxypurine. Hypoxanthine is a necessary additive in certain cells, bacteria, and parasite cultures as a substrate and nitrogen source. For example, it is commonly a required reagent in malaria culture, malaria parasite cultures, since ''Plasmodium falciparum'' requires a source of hypoxanthine for nucleic acid synthesis and energy metabolism. In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting hypoxanthine and related organic molecules, including the DNA and RNA components adenine and guanine, may have been formed extraterrestrially in outer space. The ''Pheretima, Pheretima aspergillum'' worm, used in Chinese medicine preparations, contains hypoxanthine. Reactions It is one of the products of the ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theobromine
Theobromine, also known as xantheose, is the principal alkaloid of ''Theobroma cacao'' (cacao plant). Theobromine is slightly water-soluble (330 mg/L) with a bitter taste. In industry, theobromine is used as an additive and precursor to some cosmetics. It is found in chocolate, as well as in a number of other foods, including tea (''Camellia sinensis''), some American hollies ( yaupon and guayusa) and the kola nut. It is a white or colourless solid, but commercial samples can appear yellowish. Theobromine, a metabolite of caffeine, is processed in the liver into xanthine and methyluric acid, peaks in the blood 2–3 hours after ingestion due to its fat solubility, and primarily acts by inhibiting adenosine receptors with minor phosphodiesterase inhibition. It is a mild heart stimulant and bronchodilator in humans with limited central nervous system effects. It can be toxic or fatal to animals like dogs and cats due to their slower metabolism of the compound. Structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theophylline
Theophylline, also known as 1,3-dimethylxanthine, is a drug that inhibits phosphodiesterase and blocks adenosine receptors. It is used to treat chronic obstructive pulmonary disease (COPD) and asthma. Its pharmacology is similar to other methylxanthine drugs (e.g., theobromine and caffeine). Trace amounts of theophylline are naturally present in tea, coffee, chocolate, yerba maté, guarana, and kola nut. The name 'theophylline' derives from "Thea"—the former genus name for tea + Legacy Greek φύλλον (phúllon, "leaf") + -ine. Medical uses The main actions of theophylline involve: * relaxing bronchial smooth muscle * increasing heart muscle contractility and efficiency (positive inotrope) * increasing heart rate (positive chronotropic) * increasing blood pressure * increasing renal blood flow * anti-inflammatory effects * central nervous system stimulatory effect, mainly on the medullary respiratory center The main therapeutic uses of theophylline are for treat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uric Acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the Chemical formula, formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. Hyperuricemia, High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones. Chemistry Uric acid was first isolated from kidney stones in 1776 by Swedish chemist Carl Wilhelm Scheele. In 1882, the Ukrainian chemist Ivan Horbaczewski first synthesized uric acid by melting urea with glycine. Uric acid displays lactam–lactim tautomerism. Uric acid crystallizes in the lactam form, with computational chemistry also indicating that tautomer to be the most stable. Uric acid is a diprotic acid with pKa, p''K''a1 = 5.4 and p''K''a2 =&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Degradation
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. Both adenine and guanine are derived from the nucleotide inosine monophosphate (IMP), which is the first compound in the pathway to have a completely formed purine ring system. IMP Inosine monophosphate is synthesized on a pre-existing ribose-phosphate through a complex pathway (as shown in the figure on the right). The source of the carbon and nitrogen atoms of the purine ring, 5 and 4 respectively, come from multiple sources. The amino acid glycine contributes all its carbon (2) and nitrogen (1) atoms, with additional nitrogen atoms from glutamine (2) and aspartic acid (1), and additional carbon atoms from formyl groups (2), which are transferred from the coenzyme tetrahydrofolate as 10-formyltetrahydrofolate, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (genetics)
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called ''primary'' or ''canonical''. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, crea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine
Adenosine (symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9- glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life on Earth. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias. Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12) and as a radical in the radical SAM enzymes. Medical uses Supraventricular tachycardia In individuals with supraventricular tachycardia (SVT), adenosine is a first line trea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs, such as liver and kidney, and in various seafoods, high-fructose beverages, alcohol, and yeast products. Examples of high-purine food sources include anchovies, sardines, liver, beef, kidneys, brains, monkfish, dried mackerel, and shrimp. Foods particularly rich in hypoxanthine, adenine, and guanine lead to higher blood levels of uric acid. Foods having more than 200 mg of hypoxanthine per 100 g, particularly animal and fish meats containing hypoxanthine as more than 50% of total purines, are more likely to increase uri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |