|
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids, where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-hydroxypurine. Hypoxanthine is a necessary additive in certain cells, bacteria, and parasite cultures as a substrate and nitrogen source. For example, it is commonly a required reagent in malaria parasite cultures, since '' Plasmodium falciparum'' requires a source of hypoxanthine for nucleic acid synthesis and energy metabolism. In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting hypoxanthine and related organic molecules, including the DNA and RNA components adenine and guanine, may have been formed extraterrestrially in outer space. The '' Pheretima aspergillum'' worm, used in Chinese medicine preparations, contains hypoxanthine. Reactions It is one of the products of the action of xanthine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoxanthine-guanine Phosphoribosyltransferase
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) is an enzyme encoded in humans by the ''HPRT1'' gene. HGPRT is a transferase that catalyzes conversion of hypoxanthine to inosine monophosphate and guanine to guanosine monophosphate. This reaction transfers the 5-phosphoribosyl group from 5-phosphoribosyl 1-pyrophosphate (PRPP) to the purine. HGPRT plays a central role in the generation of purine nucleotides through the purine salvage pathway. Function HGPRT catalyzes the following reactions: HGPRTase functions primarily to salvage purines from degraded DNA to reintroduce into purine synthetic pathways. In this role, it catalyzes the reaction between guanine and phosphoribosyl pyrophosphate (PRPP) to form GMP, or between hypoxanthine and phosphoribosyl pyrophosphate (PRPP) to form inosine monophosphate. Substrates and inhibitors Comparative homology modelling of this enzyme in '' L. donovani'' suggest that among all of the computationally screened compounds, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotide Salvage
A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides (purine and pyrimidine) are synthesized from intermediates in their degradative pathway. Nucleotide salvage pathways are used to recover bases and nucleosides that are formed during degradation of RNA and DNA. This is important in some organs because some tissues cannot undergo de novo synthesis. The salvaged products can then be converted back into nucleotides. Salvage pathways are targets for drug development, one family being called antifolates. A number of other biologically-important substances, like methionine and nicotinate, have their own salvage pathways to recycle parts of the molecule. Substrates The nucleotide salvage pathway requires distinct substrates: Pyrimidines Uridine phosphorylase or pyrimidine-nucleoside phosphorylase sub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Degradation
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. Both adenine and guanine are derived from the nucleotide inosine monophosphate (IMP), which is the first compound in the pathway to have a completely formed purine ring system. IMP Inosine monophosphate is synthesized on a pre-existing ribose-phosphate through a complex pathway (as shown in the figure on the right). The source of the carbon and nitrogen atoms of the purine ring, 5 and 4 respectively, come from multiple sources. The amino acid glycine contributes all its carbon (2) and nitrogen (1) atoms, with additional nitrogen atoms from glutamine (2) and aspartic acid (1), and additional carbon atoms from formyl groups (2), which are transferred from the coenzyme tetrahydrofolate as 10-formyltetrahydrofolate, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
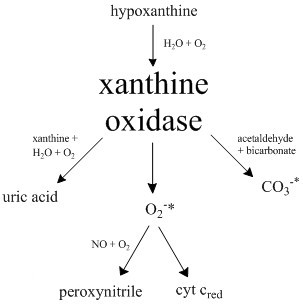
Xanthine Oxidoreductase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9- glycosidic bond. It was discovered in 1965 in analysis of RNA transferase. Inosine is commonly found in tRNAs and is essential for proper translation of the genetic code in wobble base pairs. Knowledge of inosine metabolism has led to advances in immunotherapy in recent decades. Inosine monophosphate is oxidised by the enzyme inosine monophosphate dehydrogenase, yielding xanthosine monophosphate, a key precursor in purine metabolism. Mycophenolate mofetil is an anti-metabolite, anti-proliferative drug that acts as an inhibitor of inosine monophosphate dehydrogenase. It is used in the treatment of a variety of autoimmune diseases including granulomatosis with polyangiitis because the uptake of purine by actively dividing B cells can exceed 8 times that of normal body cells, and, therefore, this set of white cells (which cannot operate purine salva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, cauliflo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pheretima
''Pheretima'' is a genus of earthworms found mostly in New Guinea and parts of Southeast Asia. Species belonging to the genus Pheretima have a clitellum, which is a band of glandular tissue present on segments 14 to 16. Individuals are hermaphroditic and reproduction can be either sexual or parthenogenetic. Female genital pores lie on the ventral surface of segment 14. A pair of male genital pores is situated ventrally on segment 18. Genital papillae may also be present ventrally. As with all earthworms, development of young is without a larval stage and takes place in cocoons. Pheretima are generally nocturnal, like most earthworms, and have an aversion to light. They come out only at night, and feed and reproduce only at night. Similar to most earthworms, they must keep their body surface wet to respire. They are also called farmer's friend as they help in making the soil porous for easy irrigation. Similar genera include ''Amynthas'', '' Archipheretima'', '' Duplodicodril ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine
Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine. Xanthine is a product on the pathway of purine degradation. * It is created from guanine by guanine deaminase. * It is created from hypoxanthine by xanthine oxidoreductase. * It is also created from xanthosine by purine nucleoside phosphorylase. Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme. Use and manufacturing Xanthine is used as a drug precursor for human and animal medications, and is manufactured as a pesticide ingredient. Clinical significance Derivatives of xanthine (known collectively as xanthines) are a group of alkaloids commonly used for their effects as mild stimulants and as bronchodilators, notably in the treatment of asthma or infl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
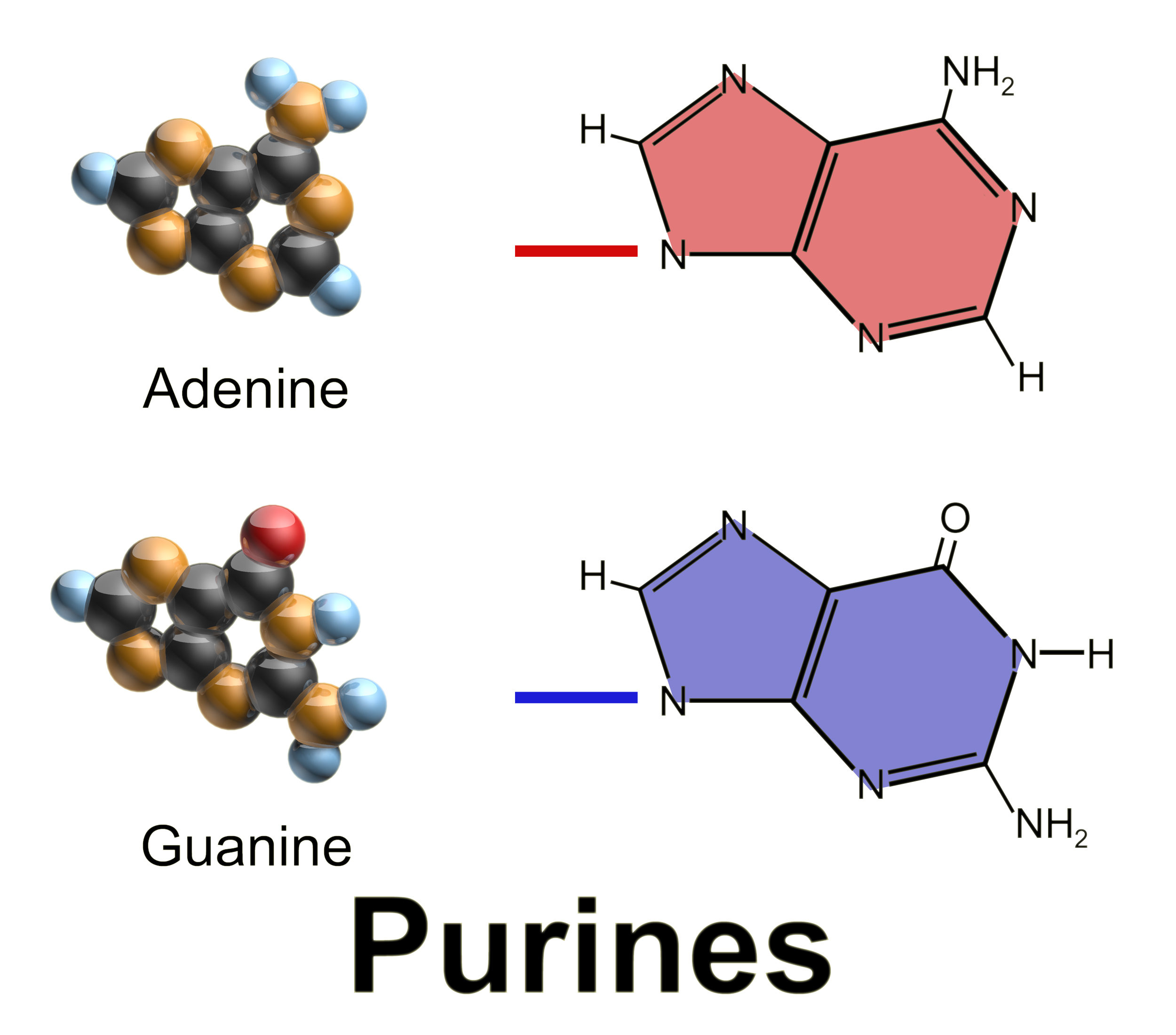
Guanine
Guanine () ( symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine- imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar. Properties Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has two tautomeric forms, the major keto form (see figures) and rare enol form. It binds to cytosine through three hydrogen bonds. In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors. Guanine has the C-6 carbonyl group that acts as the hydrogen bond acceptor, while a g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases. In the human body, deamination takes place primarily in the liver, however it can also occur in the kidney. In situations of excess protein intake, deamination is used to break down amino acids for energy. The amino group is removed from the amino acid and converted to ammonia. The rest of the amino acid is made up of mostly carbon and hydrogen, and is recycled or oxidized for energy. Ammonia is toxic to the human system, and enzymes convert it to urea or uric acid by addition of carbon dioxide molecules (which is not considered a deamination process) in the urea cycle, which also takes place in the liver. Urea and uric acid can safely diffuse into the blood and then be excreted in urine. Deamination reactions in DNA Cytosine Spontaneous deamination is the hydrolysis reaction of cytosine into uracil, releasing ammonia in the process. This can occur in vitro th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inosine Monophosphate
Inosinic acid or inosine monophosphate (IMP) is a nucleotide (that is, a nucleoside monophosphate). Widely used as a flavor enhancer, it is typically obtained from chicken byproducts or other meat industry waste. Inosinic acid is important in metabolism. It is the ribonucleotide of hypoxanthine and the first nucleotide formed during the synthesis of purine nucleotides. It can also be formed by the deamination of adenosine monophosphate by AMP deaminase. It can be hydrolysed to inosine. The enzyme deoxyribonucleoside triphosphate pyrophosphohydrolase, encoded by YJR069C in ''Saccharomyces cerevisiae'' and containing (d)ITPase and (d)XTPase activities, hydrolyzes inosine triphosphate (ITP) releasing pyrophosphate and IMP. Important derivatives of inosinic acid include the purine nucleotides found in nucleic acids and adenosine triphosphate, which is used to store chemical energy in muscle and other tissues. In the food industry, inosinic acid and its salts such as disodium inosi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |