|
Wharton Reaction
The Wharton olefin synthesis or the Wharton reaction is a chemical reaction that involves the reduction of α,β-epoxy ketones using hydrazine to give allylic alcohols. This reaction, introduced in 1961 by P. S. Wharton, is an extension of the Wolff–Kishner reduction. The general features of this synthesis are: 1) the epoxidation of α,β-unsaturated ketones is achieved usually in basic conditions using hydrogen peroxide solution in high yield; 2) the epoxy ketone is treated with 2–3 equivalents of a hydrazine hydrate in presence of stochiometry, substoichiometric amounts of acetic acid. This reaction occurs rapidly at room temperature with the evolution of nitrogen and the formation of an allylic alcohol. It can be used to synthesize compounds. Wharton's initial procedure has been improved. Mechanism and scope The Reaction mechanism, mechanism of the Wharton reaction begins with reaction of the ketone (1) with hydrazine to form a hydrazone (2). Rearrangement of the hydraz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
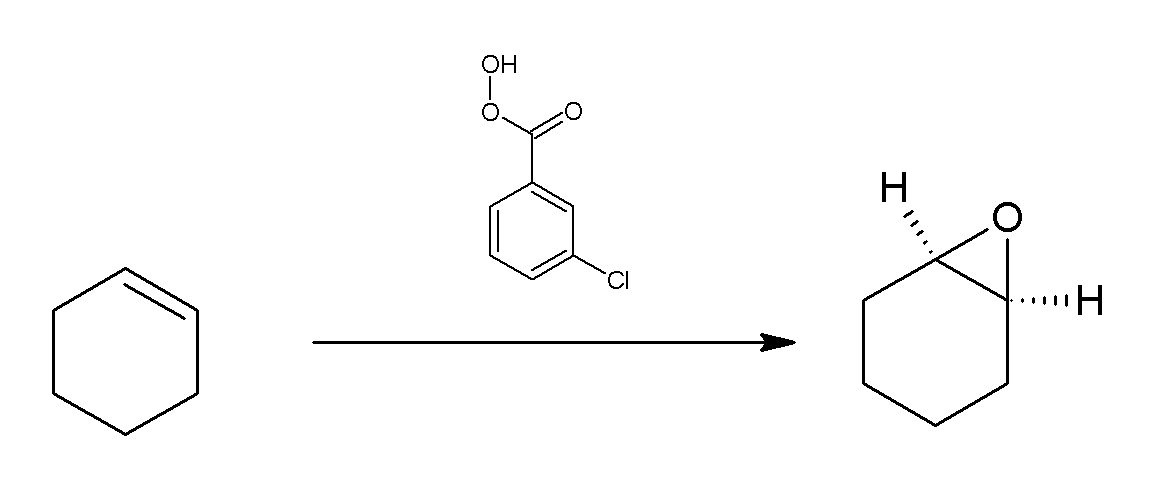
Meta-Chloroperoxybenzoic Acid
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-Chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so one can extract the acid impurity by careful control of pH. The purified material is reasonably stable against de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grob Fragmentation
In chemistry Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ..., a Grob fragmentation is an elimination reaction that breaks a neutral aliphatic chain into three fragments: a cation, positive ion spanning atoms 1 and 2 (the "electrofuge"), an unsaturated hydrocarbon, unsaturated neutral fragment spanning positions 3 and 4, and a anion, negative ion (the "nucleofuge") comprising the rest of the chain. For example, the positive ion may be a carbenium ion, carbenium, carbonium ion, carbonium or acylium ion; the neutral fragment could be an alkene, alkyne, or imine; and the negative fragment could be a tosyl or hydroxyl ion: The reaction is named for the Swiss chemist . Alternately, atom 1 could begin as an anion, in which case it becomes neutral rather than going from neutral to cation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eschenmoser Fragmentation
The Eschenmoser fragmentation, first published in 1967, is the chemical reaction of α,β-epoxyketones (1) with aryl sulfonylhydrazines (2) to give alkynes (3) and carbonyl compounds (4). The reaction is named after the Swiss chemist Albert Eschenmoser, who devised it in collaboration with an industrial research group of Günther Ohloff, and applied it to the production of muscone and related macrocyclic musks. The reaction is also sometimes known as the Eschenmoser–Ohloff fragmentation or the Eschenmoser–Tanabe fragmentation as Masato Tanabe independently published an article on the reaction the same year. The general formula of the fragmentation using ''p''-toluenesulfonylhydrazide is: :: Several examples exist in the literature, and the reaction is also carried out on industrial scale. Reaction mechanism The mechanism of the Eschenmoser fragmentation begins with the condensation of an α,β-epoxyketone (1) with an aryl sulfonylhydrazine (2) to afford the intermediate hydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal diol, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis (both semisynthesis and total synthesis) and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients. Within the field of organic chemistry, the definition of natural products is usually restricted to organic compounds isolated from natural sources that are produced by the pathways of primary or secondary metabolism. Within the field of medicinal chemistry, the definition is often further restricted to secondary metabolites. Secondary metabolites (or specialized metabolites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticancer
An anticarcinogen (also known as a carcinopreventive agent) is a substance that counteracts the effects of a carcinogen or inhibits the development of cancer. Anticarcinogens are different from anticarcinoma agents (also known as anticancer or anti-neoplastic agents) in that anticarcinoma agents are used to selectively destroy or inhibit cancer cells ''after'' cancer has developed. Interest in anticarcinogens is motivated primarily by the principle that it is preferable to prevent disease (preventive medicine) than to have to treat it ( rescue medicine). In theory, anticarcinogens may act via different mechanisms including enhancement of natural defences against cancer, deactivation of carcinogens, and blocking the mechanisms by which carcinogens act (such as free radical damage to DNA). Confirmation that a substance possesses anticarcinogenic activity requires extensive ''in vitro'', ''in vivo'', and clinical investigation. Health claims for anticarcinogens are regulated by v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E And Z
E, or e, is the fifth letter and the second vowel letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''e'' (pronounced ); plural ''ees'', ''Es'' or ''E's''. It is the most commonly used letter in many languages, including Czech, Danish, Dutch, English, French, German, Hungarian, Latin, Latvian, Norwegian, Spanish, and Swedish. History The Latin letter 'E' differs little from its source, the Greek letter epsilon, 'Ε'. This in turn comes from the Semitic letter '' hê'', which has been suggested to have started as a praying or calling human figure ('' hillul'' 'jubilation'), and was most likely based on a similar Egyptian hieroglyph that indicated a different pronunciation. In Semitic languages, Semitic, the letter represented (and in foreign words); in Greek language, Greek, ''hê'' became the letter epsilon, used to represent . The various forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open Chain Compound
In chemistry, an open-chain compound (also spelled as open chain compound) or acyclic compound (Greek prefix "α", ''without'' and "κύκλος", ''cycle'') is a compound with a linear structure, rather than a cyclic one. An open-chain compound having no side chains is called a straight-chain compound (also spelled as straight chain compound). Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic, with the latter often classified as aromatic. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix ''n-'' denotes the straight-chain isomer; for example, ''n''-butane is straight-chain butane, whereas ''i''-butane is isobutane. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring's closure involves a C-C bond. Having no rings (aromatic or otherwise), all open-chain compounds are aliphatic. Typically in bioc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when performi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diimide
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene. Synthesis A traditional route to diimide involves oxidation of hydrazine with hydrogen peroxide or air. Alternatively the hydrolysis of diethyl azodicarboxylate or azodicarbonamide affords diimide: :(NCOOH)2 → (NH)2 + 2 CO2 Nowadays, diimide is generated by thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide. Because of its instability, diimide is generated and used ''in-situ''. A mixture of both the ''cis'' (''Z-'') and ''trans'' (''E-'') isomers is produced. Both isomers are unstable, and they undergo a slow interconversion. The ''trans'' isomer is more stable, but the ''cis'' isomer is the one that reacts with unsaturated substrates, therefore the equilibrium between them shi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |