Grob Fragmentation on:
[Wikipedia]
[Google]
[Amazon]
In  The reaction is named for the Swiss chemist .
Alternately, atom 1 could begin as an anion, in which case it becomes neutral rather than going from neutral to cationic.
The reaction is named for the Swiss chemist .
Alternately, atom 1 could begin as an anion, in which case it becomes neutral rather than going from neutral to cationic.
 Albert Eschenmoser in 1952 investigated the base catalysed fragmentation of certain beta hydroxy ketones:
Albert Eschenmoser in 1952 investigated the base catalysed fragmentation of certain beta hydroxy ketones:
 The original work by Grob (1955) concerns the formation of
The original work by Grob (1955) concerns the formation of  According to reviewers Prantz and Mulzer (2010), the name Grob fragmentation was chosen "in more or less glaring disregard of the earlier contributions".
According to reviewers Prantz and Mulzer (2010), the name Grob fragmentation was chosen "in more or less glaring disregard of the earlier contributions".
 In this reaction, diastereoselective reduction of the ketone 1 with sodium borohydride yields
In this reaction, diastereoselective reduction of the ketone 1 with sodium borohydride yields
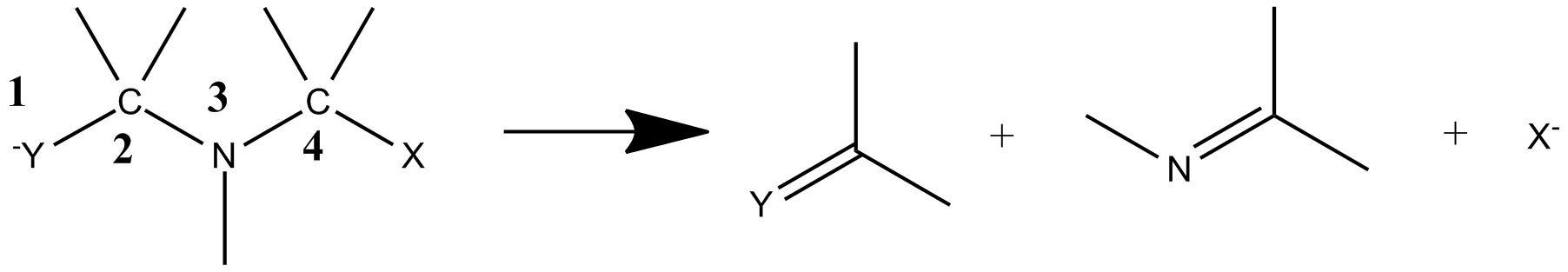 3-aza-Grob fragmentation can proceed with several different nucleofuges. The
3-aza-Grob fragmentation can proceed with several different nucleofuges. The  The scope of the reaction has been found to cover THF and tetrahydrothiophene protecting groups using various hydride agents.
The scope of the reaction has been found to cover THF and tetrahydrothiophene protecting groups using various hydride agents.
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a Grob fragmentation is an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
that breaks a neutral aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
chain into three fragments: a positive ion spanning atoms 1 and 2 (the " electrofuge"), an unsaturated neutral fragment spanning positions 3 and 4, and a negative ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(the " nucleofuge") comprising the rest of the chain.
For example, the positive ion may be a carbenium
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In older literature the name carbonium ion was used for this class, but now it refers exclusivel ...
, carbonium
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms.
The nex ...
or acylium ion
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC n ...
; the neutral fragment could be an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, or imine; and the negative fragment could be a tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on s ...
or hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
ion:
History
An early instance of fragmentation is thedehydration
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mil ...
of di(''tert''-butyl)methanol yielding 2-methyl-2-butene
2-Methyl-2-butene, 2m2b, 2-methylbut-2-ene, also beta-isoamylene is an alkene hydrocarbon with the molecular formula C5H10.
Used as a free radical scavenger in trichloromethane (chloroform) and dichloromethane (methylene chloride).
John Snow, th ...
and isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Productio ...
, a reaction described in 1933 by Frank C. Whitmore
Frank Clifford Whitmore (October 1, 1887 – June 24, 1947), nicknamed "Rocky", was a prominent chemist who submitted significant evidence for the existence of carbocation mechanisms in organic chemistry.
He was born in 1887 in the town of North ...
. This reaction proceeds by formation of a secondary carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
followed by a rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another ...
to a more stable tertiary carbocation and elimination of a ''t''-butyl cation:
1,5-hexadiene
1,5-Hexadiene is the organic compound with the formula (CH)(CH=CH). It is a colorless, volatile liquid. It is used as a crosslinking agent and precursor to a variety of other compounds.
Synthesis
1,5-Hexadiene is produced commercially by the eth ...
from ''cis''- or ''trans''-1,4-dibromocyclohexane by sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
metal:
Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
varies with reactant and reaction conditions with the fragmentation taking place in a concerted reaction or taking place in two steps with a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
ic intermediate when the nucleofuge leaves first or taking place in two steps with an anionic intermediate when the electrofuge leaves first. The carbanionic pathway is more common and is facilitated by the stability of the cation formed and the leaving group ability of the nucleofuge. With cyclic substrates, the preferred geometry of elimination is for the sigma bond that drives out the leaving group to being anti to it, analogous to the conformational orientation in the E2 mechanism of elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
s.
Examples
Thapsigargin from Wieland–Miescher ketone
An example of a Grob-like fragmentation inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
is the expansion of the Wieland–Miescher ketone to thapsigargin:
 In this reaction, diastereoselective reduction of the ketone 1 with sodium borohydride yields
In this reaction, diastereoselective reduction of the ketone 1 with sodium borohydride yields alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
2, which is functionalized to the mesylate
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group ...
3 with mesyl chloride in pyridine. The selectivity of the initial reduction of ketone 1 is a result of borohyride approaching from the bottom face to avoid steric clash with the axial methyl group. Then reduction of the enone to allyl alcohol 4 with tri-tert-butoxyaluminum hydride, tri-''tert''-butoxyaluminium hydride in tetrahydrofuran followed by hydroboration with borane in THF yields the borane 5 (only one substituent displayed for clarity). The diastereoselectivity of the hydroboration is a result of two factors: avoidance of the axial methyl group as well as axial hydride addition to avoid a twist-boat conformation in the transition state. The Grob fragmentation to 6 takes place with sodium methoxide in methanol at reflux. A methoxide group attacks the boron atom giving a borate complex which fragments. As each boron atom can hold three substrate molecules (R), the ultimate boron byproduct is trimethyl borate. As seen in 6, the mesylate being in the equatorial position allows its sigma star orbital to align ideally with the sigma bond drawn, allowing for the correct olefin geometry seen in 7.
Another example is an epoxy alcohol fragmentation reaction as part of the Holton Taxol total synthesis#Synthesis AB ring, Holton Taxol total synthesis.
aza-Grob fragmentation
3-aza-Grob fragmentation is variation which takes place when an electrofuge and nucleofuge are situated at positions 1 and 5 on a secondary or tertiary amine chain with the nitrogen at the 3 position. The reaction products are an electrofugal fragment, an imine, and a nucleofugal fragment (such as analcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
).
 3-aza-Grob fragmentation can proceed with several different nucleofuges. The
3-aza-Grob fragmentation can proceed with several different nucleofuges. The reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
has been reported to begin with the reduction of an ether protected amide to form a secondary alcohol. Fragmentation then takes place in a concerted step to form the reaction products.
 The scope of the reaction has been found to cover THF and tetrahydrothiophene protecting groups using various hydride agents.
The scope of the reaction has been found to cover THF and tetrahydrothiophene protecting groups using various hydride agents.
See also
*Eschenmoser fragmentation *Wharton reactionReferences
{{DEFAULTSORT:Grob Fragmentation Elimination reactions Name reactions