|
Electrofuge
An electrofuge is a leaving group which does not retain the bonding pair of electrons from its previous bond with another species. It can result from the heterolytic breaking of covalent bonds. After this reaction an electrofuge may possess either a positive or a neutral charge; this is governed by the nature of the specific reaction. An example would be the loss of H+ from a molecule of benzene during nitration. The word 'electrofuge' is commonly found in older literature, but its use in contemporary organic chemistry is now uncommon. See also *Nucleofuge *Nucleophile In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ... * Electrophile References * . Organic chemistry {{Chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
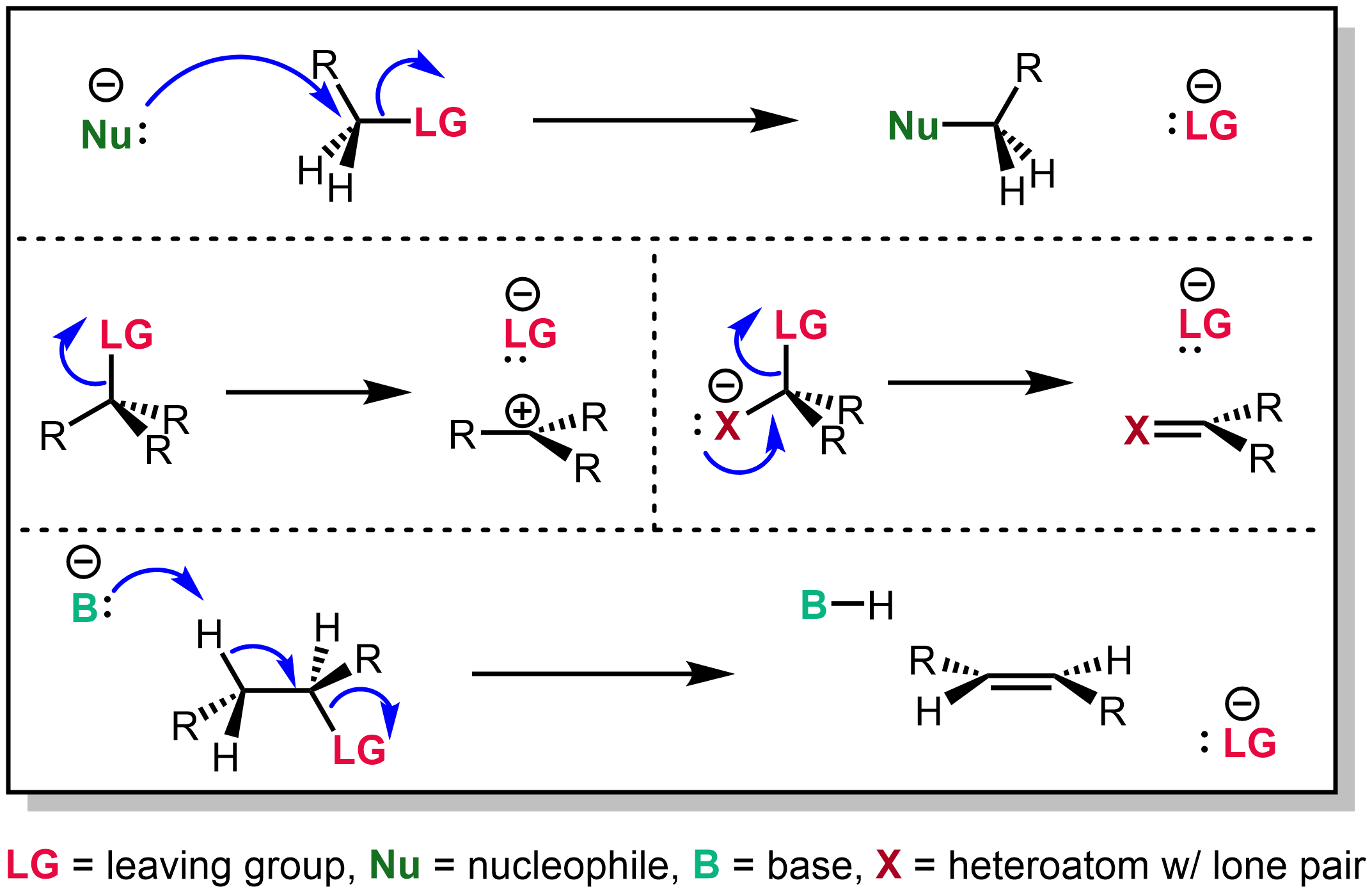
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleofuge
A nucleofuge (derived from ''nucleo'', nucleus, the positive part in an atom and fuge, ''fugitive'', to run away, to escape) is a leaving group which retains the lone pair from its previous bond with another species. For example, in the SN2 mechanism a nucleophile attacks an organic compound containing the nucleofuge (the bromo group) which simultaneously breaks the bond with the nucleofuge. After a reaction nucleofuges may contain either a negative or a neutral charge; this is governed by the nature of the specific reaction. The word 'nucleofuge' is commonly found in older literature, but its use is less common in current literature in which the word leaving group dominates. See also * Electrofuge *Nucleophile In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ... * Electroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reactions i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |